Possible precision medicine implications from genetic testing using combined detection of sequence and intragenic copy number variants in a large cohort with childhood epilepsy
- PMID: 31440721
- PMCID: PMC6698688
- DOI: 10.1002/epi4.12348
Possible precision medicine implications from genetic testing using combined detection of sequence and intragenic copy number variants in a large cohort with childhood epilepsy
Abstract
Objective: Molecular genetic etiologies in epilepsy have become better understood in recent years, creating important opportunities for precision medicine. Building on these advances, detailed studies of the complexities and outcomes of genetic testing for epilepsy can provide useful insights that inform and refine diagnostic approaches and illuminate the potential for precision medicine in epilepsy.
Methods: We used a multi-gene next-generation sequencing (NGS) panel with simultaneous sequence and exonic copy number variant detection to investigate up to 183 epilepsy-related genes in 9769 individuals. Clinical variant interpretation was performed using a semi-quantitative scoring system based on existing professional practice guidelines.
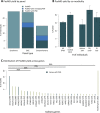
Results: Molecular genetic testing provided a diagnosis in 14.9%-24.4% of individuals with epilepsy, depending on the NGS panel used. More than half of these diagnoses were in children younger than 5 years. Notably, the testing had possible precision medicine implications in 33% of individuals who received definitive diagnostic results. Only 30 genes provided 80% of molecular diagnoses. While most clinically significant findings were single-nucleotide variants, ~15% were other types that are often challenging to detect with traditional methods. In addition to clinically significant variants, there were many others that initially had uncertain significance; reclassification of 1612 such variants with parental testing or other evidence contributed to 18.5% of diagnostic results overall and 6.1% of results with precision medicine implications.
Significance: Using an NGS gene panel with key high-yield genes and robust analytic sensitivity as a first-tier test early in the diagnostic process, especially for children younger than 5 years, can possibly enable precision medicine approaches in a significant number of individuals with epilepsy.
Keywords: clinical management; copy number variant; diagnostic genetic testing; next‐generation sequencing panel; precision medicine; variant of uncertain significance.
Conflict of interest statement
Rebecca Truty, Nila Patil, Ali Entezam, Edward D. Esplin, Amy Fuller, Michelle Hogue, Britt Johnson, Amirah Khouzam, Yuya Kobayashi, Rachel Lewis, Keith Nykamp, Darlene Riethmaier, Jody Westbrook, Michelle Zeman, Robert L. Nussbaum, and Swaroop Aradhya are full‐time employees of Invitae, a genetic testing company. Dr. Raman Sankar and Dr. Joseph Sullivan are advisors to Invitae. The other authors have no conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


References
-
- Thomas RH, Berkovic SF. The hidden genetics of epilepsy – a clinically important new paradigm. Nat Rev Neurol. 2014;10:283–92. - PubMed
-
- Symonds JD, Zuberi SM, Johnson MR. Advances in epilepsy gene discovery and implications for epilepsy diagnosis and treatment. Curr Opin Neurol. 2017;30:193–99. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

