Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro
- PMID: 31441218
- PMCID: PMC6980227
- DOI: 10.1111/vop.12704
Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro
Abstract
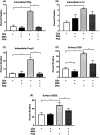
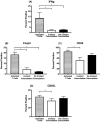
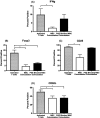
Equine recurrent uveitis (ERU) is an immune-mediated disease causing repeated or persistent inflammatory episodes which can lead to blindness. Currently, there is no cure for horses with this disease. Mesenchymal stem cells (MSCs) are effective at reducing immune cell activation in vitro in many species, making them a potential therapeutic option for ERU. The objectives of this study were to define the lymphocyte phenotype of horses with ERU and to determine how MSCs alter T-cell phenotype in vitro. Whole blood was taken from 7 horses with ERU and 10 healthy horses and peripheral blood mononuclear cells were isolated. The markers CD21, CD3, CD4, and CD8 were used to identify lymphocyte subsets while CD25, CD62L, Foxp3, IFNγ, and IL10 were used to identify T-cell phenotype. Adipose-derived MSCs were expanded, irradiated (to control proliferation), and incubated with CD4+ T-cells from healthy horses, after which lymphocytes were collected and analyzed via flow cytometry. The percentages of T-cells and B-cells in horses with ERU were similar to normal horses. However, CD4+ T-cells from horses with ERU expressed higher amounts of IFNγ indicating a pro-inflammatory Th1 phenotype. When co-incubated with MSCs, activated CD4+ T-cells reduced expression of CD25, CD62L, Foxp3, and IFNγ. MSCs had a lesser ability to decrease activation when cell-cell contact or prostaglandin signaling was blocked. MSCs continue to show promise as a treatment for ERU as they decreased the CD4+ T-cell activation phenotype through a combination of cell-cell contact and prostaglandin signaling.
Keywords: activated CD4+ T-cells; equine recurrent uveitis; immunomodulation; mesenchymal stem cells.
© 2019 The Authors. Veterinary Ophthalmology published by Wiley Periodicals, Inc. on behalf of American College of Veterinary Ophthalmologists.
Figures


References
-
- Schwink KL. Equine uveitis. Vet Clin North Am Equine Pract. 1992;8:557‐574. - PubMed
-
- Barnett KC. Equine periodic ophthalmia: a continuing aetiological riddle. Equine Vet J. 1987;19:90‐91. - PubMed
-
- Witmer R. Periodic ophthalmia in horses. Am J Ophthalmol. 1954;37:243‐253. - PubMed
-
- Dwyer A. Visual prognosis in horses with uveitis. American Society of Veterinary Ophthalmology Annual Meeting. 1998.
-
- Gilger BC, Deeg C. Chapter 8 ‐ Equine recurrent uveitis In: Gilger BC. ed. Equine Ophthalmology, 2nd edn Saint Louis, MO: W.B. Saunders; 2011:317‐349.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

