Loss of Ataxin-1 Potentiates Alzheimer's Pathogenesis by Elevating Cerebral BACE1 Transcription
- PMID: 31442405
- PMCID: PMC6726125
- DOI: 10.1016/j.cell.2019.07.043
Loss of Ataxin-1 Potentiates Alzheimer's Pathogenesis by Elevating Cerebral BACE1 Transcription
Abstract
Expansion of CAG trinucleotide repeats in ATXN1 causes spinocerebellar ataxia type 1 (SCA1), a neurodegenerative disease that impairs coordination and cognition. While ATXN1 is associated with increased Alzheimer's disease (AD) risk, CAG repeat number in AD patients is not changed. Here, we investigated the consequences of ataxin-1 loss of function and discovered that knockout of Atxn1 reduced CIC-ETV4/5-mediated inhibition of Bace1 transcription, leading to increased BACE1 levels and enhanced amyloidogenic cleavage of APP, selectively in AD-vulnerable brain regions. Elevated BACE1 expression exacerbated Aβ deposition and gliosis in AD mouse models and impaired hippocampal neurogenesis and olfactory axonal targeting. In SCA1 mice, polyglutamine-expanded mutant ataxin-1 led to the increase of BACE1 post-transcriptionally, both in cerebrum and cerebellum, and caused axonal-targeting deficit and neurodegeneration in the hippocampal CA2 region. These findings suggest that loss of ataxin-1 elevates BACE1 expression and Aβ pathology, rendering it a potential contributor to AD risk and pathogenesis.
Keywords: Alzheimer’s disease; Aβ; BACE1; CA2; amyloid precursor protein; ataxin-1; axonal targeting; hippocampal neurogenesis; neurodegeneration; spinocerebellar ataxia type 1.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
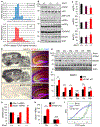
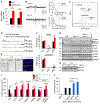

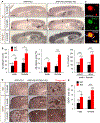

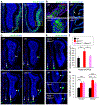

References
-
- Asher M, Johnson A, Zecevic B, Pease D, and Cvetanovic M (2016). Ataxin-1 regulates proliferation of hippocampal neural precursors. Neuroscience 322, 54–65. - PubMed
-
- Barnea G, O'Donnell S, Mancia F, Sun X, Nemes A, Mendelsohn M, and Axel R (2004). Odorant receptors on axon termini in the brain. Science 304, 1468. - PubMed
-
- Bertram L, and Tanzi RE (2012). The genetics of Alzheimer's disease. Prog Mol Biol Transl Sci 107, 79–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

