Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7
- PMID: 31442409
- PMCID: PMC6709783
- DOI: 10.1016/j.cell.2019.07.028
Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7
Abstract
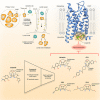
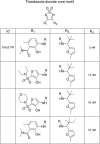
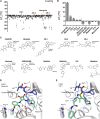
The CC chemokine receptor 7 (CCR7) balances immunity and tolerance by homeostatic trafficking of immune cells. In cancer, CCR7-mediated trafficking leads to lymph node metastasis, suggesting the receptor as a promising therapeutic target. Here, we present the crystal structure of human CCR7 fused to the protein Sialidase NanA by using data up to 2.1 Å resolution. The structure shows the ligand Cmp2105 bound to an intracellular allosteric binding pocket. A sulfonamide group, characteristic for various chemokine receptor ligands, binds to a patch of conserved residues in the Gi protein binding region between transmembrane helix 7 and helix 8. We demonstrate how structural data can be used in combination with a compound repository and automated thermal stability screening to identify and modulate allosteric chemokine receptor antagonists. We detect both novel (CS-1 and CS-2) and clinically relevant (CXCR1-CXCR2 phase-II antagonist Navarixin) CCR7 modulators with implications for multi-target strategies against cancer.
Keywords: CCR7; G protein-coupled receptors; allosteric modulation; cancer; chemokine receptors; crystal structure; lymph node metastasis; membrane proteins; structure-based drug screening.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
W.G., T.M., M.W., N.H., T.T., D.M., J.-M.V., A.G., A.K., M.G.R., J.B., and R.J.P.D. are employees of F. Hoffmann-La Roche LTD.
Figures


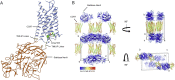





References
-
- Adams P.D., Grosse-Kunstleve R.W., Hung L.W., Ioerger T.R., McCoy A.J., Moriarty N.W., Read R.J., Sacchettini J.C., Sauter N.K., Terwilliger T.C. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1948–1954. - PubMed
-
- Balkwill F.R. The chemokine system and cancer. J. Pathol. 2012;226:148–157. - PubMed
-
- Burg J.S., Ingram J.R., Venkatakrishnan A.J., Jude K.M., Dukkipati A., Feinberg E.N., Angelini A., Waghray D., Dror R.O., Ploegh H.L., Garcia K.C. Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science. 2015;347:1113–1117. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

