3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance
- PMID: 31442885
- PMCID: PMC6745276
- DOI: 10.1016/j.biomaterials.2019.119423
3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance
Abstract
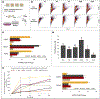
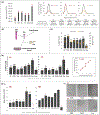
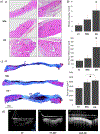
Vascularization is a crucial process during the growth and development of bone 1, yet it remains one of the main challenges in the reconstruction of large bone defects. The use of in vitro coculture of human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs) has been one of the most explored options. Both cell types secrete specific growth factors that are mutually beneficial, and studies suggested that cell-cell communication and paracrine secretion could be affected by a number of factors. However, little is known about the effect of cell patterning and the distance between cell populations on their crosstalk. In the present study, we showed that the separation and distance between ECs and MSCs populations affects angiogenesis by modulating cell-cell communication. HUVECs grown farther apart from MSCs (˃400 μm) presented characteristics of an early stage of angiogenesis (migration/proliferation). Results showed an increase in the up-regulation of VEGF, FGF-2, and ITGA3 (integrins) but a smaller fold change in the expression of VE-Cadherin and Ang-1. HUVECs were also still highly proliferative. On the contrary, HUVECs incubated closer (≤200 μm) to MSCs, showed signs of stabilization, mainly an increase in Ang-1 and VE-cadherin expression, as well as tighter monolayers. Conditioned media collected from HUVECs and MSCs grown ≤200 μm apart preferentially promoted tube formation, a later stage of angiogenesis, due in part to a significant increase in Ang-1 paracrine secretion. In addition, in groups in which fibers were printed farther apart (400 μm), cells produced EVs with a significantly increase cargo. Finally, in vivo experiment results showed an increase in blood vessels density and new bone thickness after 12 weeks of implantation in rat cranial defect, further suggesting the higher efficiency of indirect ECs/MSCs contact in prompting the release of paracrine signals that stimulate the angiogenesis of local tissues, and enhanced subsequent bone regeneration.
Keywords: 3D printing; Angiogenesis; Bone tissue engineering; EVs; Paracrine signaling.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


References
-
- Towler DA The osteogenic-angiogenic interface: Novel insights into the biology of bone formation and fracture repair. Curr. Osteoporos. Rep 6, 67–71 (2008). - PubMed
-
- Huang Y-C, Kaigler D, Rice KG, Krebsbach PH & Mooney DJ Combined Angiogenic and Osteogenic Factor Delivery Enhances Bone Marrow Stromal Cell-Driven Bone Regeneration. J. Bone Miner. Res 20, 848–857 (2004). - PubMed
-
- Radisic M, Malda J, Epping E, Geng W, Langer R & Vunjak-Novakovic G Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng 93, 332–343 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

