Metal and Polymeric Strain Gauges for Si-Based, Monolithically Fabricated Organs-on-Chips
- PMID: 31443200
- PMCID: PMC6724067
- DOI: 10.3390/mi10080536
Metal and Polymeric Strain Gauges for Si-Based, Monolithically Fabricated Organs-on-Chips
Abstract
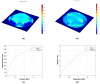
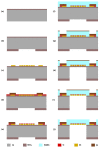
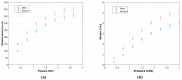
Organ-on-chip (OOC) is becoming the alternative tool to conventional in vitro screening. Heart-on-chip devices including microstructures for mechanical and electrical stimulation have been demonstrated to be advantageous to study structural organization and maturation of heart cells. This paper presents the development of metal and polymeric strain gauges for in situ monitoring of mechanical strain in the Cytostretch platform for heart-on-chip application. Specifically, the optimization of the fabrication process of metal titanium (Ti) strain gauges and the investigation on an alternative material to improve the robustness and performance of the devices are presented. The transduction behavior and functionality of the devices are successfully proven using a custom-made set-up. The devices showed resistance changes for the pressure range (0-3 kPa) used to stretch the membranes on which heart cells can be cultured. Relative resistance changes of approximately 0.008% and 1.2% for titanium and polymeric strain gauges are respectively reported for membrane deformations up to 5%. The results demonstrate that both conventional IC metals and polymeric materials can be implemented for sensing mechanical strain using robust microfabricated organ-on-chip devices.
Keywords: MEMS; PDMS; cell; membranes; organ-on-chip; silicon; strain; stress.
Conflict of interest statement
C.S., N.G. and W.F.Q.-S. are founders of the Startup Company BIOND Solutions B.V. (BI/OND), a spin-off from Delft University of Technology. G.P., R.D. and P.M.S. declare no potential conflict of interests.
Figures






References
-
- Khoshfetrat Pakazad S. A novel stretchable micro-electrode array (SMEA) design for directional stretching of cells. J. Micromech. Microeng. 2014;24:34003. doi: 10.1088/0960-1317/24/3/034003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

