Beneficial Effects of Non-Encapsulated or Encapsulated Probiotic Supplementation on Microbiota Composition, Intestinal Barrier Functions, Inflammatory Profiles, and Glucose Tolerance in High Fat Fed Rats
- PMID: 31443365
- PMCID: PMC6769526
- DOI: 10.3390/nu11091975
Beneficial Effects of Non-Encapsulated or Encapsulated Probiotic Supplementation on Microbiota Composition, Intestinal Barrier Functions, Inflammatory Profiles, and Glucose Tolerance in High Fat Fed Rats
Abstract
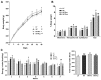
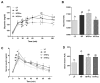
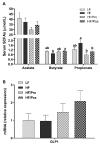
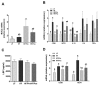
Development of obesity-associated comorbidities is related to chronic inflammation, which has been linked to gut microbiota dysbiosis. Thus, modulating gut microbiota composition could have positive effects for metabolic disorders, supporting the use of probiotics as potential therapeutics in vivo, which may be enhanced by a microencapsulation technique. Here we investigated the effects of non-encapsulated or pectin-encapsulated probiotic supplementation (Lactobacillus paracasei subsp. paracasei L. casei W8®; L. casei W8) on gut microbiota composition and metabolic profile in high-fat (HF) diet-fed rats. Four male Wistar rat groups (n = 8/group) were fed 10% low-fat, 45% HF, or HF with non-encapsulated or encapsulated L. casei W8 (4 × 107 CFU/g diet) diet for seven weeks. Microbiota composition, intestinal integrity, inflammatory profiles, and glucose tolerance were assessed. Non-encapsulated and pectin-encapsulated probiotic supplementation positively modulated gut microbiota composition in HF-fed male rats. These changes were associated with improvements in gut barrier functions and local and systemic inflammation by non-encapsulated probiotics and improvement in glucose tolerance by encapsulated probiotic treatment. Thus, these findings suggest the potential of using oral non-encapsulated or encapsulated probiotic supplementation to ameliorate obesity-associated metabolic abnormalities.
Keywords: glucose tolerance; gut microbiota; inflammation; intestinal epithelial barrier; microencapsulation; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- de La Serre C.B., Ellis C.L., Lee J., Hartman A.L., Rutledge J.C., Raybould H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

