Maternal Haplotypes in DHFR Promoter and MTHFR Gene in Tuning Childhood Acute Lymphoblastic Leukemia Onset-Latency: Genetic/Epigenetic Mother/Child Dyad Study (GEMCDS)
- PMID: 31443485
- PMCID: PMC6770441
- DOI: 10.3390/genes10090634
Maternal Haplotypes in DHFR Promoter and MTHFR Gene in Tuning Childhood Acute Lymphoblastic Leukemia Onset-Latency: Genetic/Epigenetic Mother/Child Dyad Study (GEMCDS)
Abstract
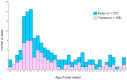
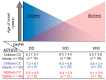
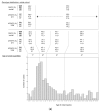
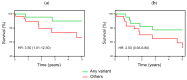
Childhood acute lymphoblastic leukemia (ALL) peaks around age 2-4, and in utero genetic epigenetic mother-fetus crosstalk might tune ALL onset during childhood life. Folate genes variably interact with vitamin status on ALL risk and prognosis. We investigated DHFR and MTHFR gene variants in 235 ALL children and their mothers to disclose their role in determining ALL onset age and survival. Pyrosequence of DHFR 19bp ins/del (rs70991108; W/D), MTHFR C677T (rs1801133; C>T), and MTHFR A1298C (rs1801131; A>C) was assessed in children and in 72% of mothers for dyad-analysis comparison. DHFR DD-children had delayed ALL onset compared to WW-children (7.5 ± 4.8 vs. 5.2 ± 3.7 years; P = 0.002) as well as MTHFR 1298 CC-children compared to AA-children (8.03 ± 4.8 vs. 5.78 ± 4.1 years; P = 0.006), and according to the strong linkage disequilibrium between MTHFR 677 T-allele and 1298C-allele, MTHFR TT-children showed early mean age of onset though not significant. Offspring of MTHFR 677 TT-mothers had earlier ALL onset compared to offspring of 677 CC-mothers (5.4 ± 3.3 vs. 7 ± 5.3 years; P = 0.017). DHFR/MTHFR 677 polymorphism combination influenced onset age by comparing DD/CC vs. WW/TT children (8.1 ± 5.7 vs. 4.7 ± 2.1 years; P = 0.017). Moreover, mother-child genotype combination gave 5.5-years delayed onset age in favor of DD-offspring of 677 CC-mothers vs. WW-offspring of 677 TT-mothers, and it was further confirmed including any D-carrier children and any 677 T-carrier mothers (P = 0.00052). Correction for multiple comparisons maintained statistical significance for DHFR ins/del and MTHFR A1298C polymorphisms. Unexpectedly, among the very-early onset group (<2.89 years; 25th), DD-genotype inversely clustered in children and mothers (4.8% vs. 23.8% respectively), and accordingly ALL offspring of homozygous DD-mothers had increased risk to have early-onset (adjusted OR (odds ratio) = 3.08; 1.1-8.6; P = 0.03). The opposite effect DHFR promoter variant has in tuning ALL onset-time depending on who is the carrier (i.e., mother or child) might suggest a parent-origin-effect of the D-allele or a two-faced epigenetic role driven by unbalanced folate isoform availability during the in-utero leukemogenesis responsible for the wide postnatal childhood ALL latency.
Keywords: ALL onset; Childhood Acute Lymphoblastic Leukemia; DHFR; MTHFR; MTX; epigenetics; folate; mother-fetus in utero crosstalk; parent-origin effect (POE); polymorphism/gene variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Methylene tetrahydrofolate reductase gene polymorphism in Egyptian children with acute lymphoblastic leukemia.Blood Coagul Fibrinolysis. 2010 Jan;21(1):28-34. doi: 10.1097/MBC.0b013e32833135e9. Blood Coagul Fibrinolysis. 2010. PMID: 19923983
-
Polymorphisms of the folate metabolizing enzymes: Association with SLE susceptibility and in silico analysis.Gene. 2017 Dec 30;637:161-172. doi: 10.1016/j.gene.2017.09.037. Epub 2017 Sep 22. Gene. 2017. PMID: 28943344
-
The association of methylenetetrahydrofolate reductase genotypes with the risk of childhood leukemia in Taiwan.PLoS One. 2015 Mar 20;10(3):e0119776. doi: 10.1371/journal.pone.0119776. eCollection 2015. PLoS One. 2015. PMID: 25793509 Free PMC article.
-
A meta-analysis of MTHFR C677T and A1298C polymorphisms and risk of acute lymphoblastic leukemia in children.Pediatr Blood Cancer. 2012 Apr;58(4):513-8. doi: 10.1002/pbc.23137. Epub 2011 Apr 14. Pediatr Blood Cancer. 2012. PMID: 21495160 Review.
-
MTHFR-C677T Gene Polymorphism and Susceptibility to Acute Lymphoblastic Leukemia in Children: A Meta-Analysis.Crit Rev Eukaryot Gene Expr. 2020;30(2):125-136. doi: 10.1615/CritRevEukaryotGeneExpr.2020033468. Crit Rev Eukaryot Gene Expr. 2020. PMID: 32558492 Review.
Cited by
-
Epigenetic role of LINE-1 methylation and key genes in pregnancy maintenance.Sci Rep. 2024 Feb 8;14(1):3275. doi: 10.1038/s41598-024-53737-2. Sci Rep. 2024. PMID: 38332006 Free PMC article.
-
Sex/Gender-Specific Imbalance in CVD: Could Physical Activity Help to Improve Clinical Outcome Targeting CVD Molecular Mechanisms in Women?Int J Mol Sci. 2020 Feb 21;21(4):1477. doi: 10.3390/ijms21041477. Int J Mol Sci. 2020. PMID: 32098263 Free PMC article. Review.
-
COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males?Int J Mol Sci. 2020 May 14;21(10):3474. doi: 10.3390/ijms21103474. Int J Mol Sci. 2020. PMID: 32423094 Free PMC article.
-
LINE-1 Methylation sustains telomere length in pregnant women: effects on pregnancy failure.Clin Epigenetics. 2025 Jul 23;17(1):130. doi: 10.1186/s13148-025-01937-6. Clin Epigenetics. 2025. PMID: 40702545 Free PMC article.
-
Genetics and Epigenetics of One-Carbon Metabolism Pathway in Autism Spectrum Disorder: A Sex-Specific Brain Epigenome?Genes (Basel). 2021 May 20;12(5):782. doi: 10.3390/genes12050782. Genes (Basel). 2021. PMID: 34065323 Free PMC article. Review.
References
-
- WHO/Europe Incidence of Childhood Leukaemia. [(accessed on 3 May 2019)]; Available online: http://www.euro.who.int/en/home.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

