A Divergent Alkyne Diol Directs [2 + 2] Photoreactivity in the Solid State: Cocrystal, Supramolecular Catalysis, and Sublimation Effects
- PMID: 31443541
- PMCID: PMC6749457
- DOI: 10.3390/molecules24173059
A Divergent Alkyne Diol Directs [2 + 2] Photoreactivity in the Solid State: Cocrystal, Supramolecular Catalysis, and Sublimation Effects
Abstract
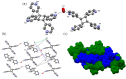
2-butyne-1,4-diol (1,4-bd) is used as a divergent ditopic template that directs trans-1,2-bis (n-pyridyl) ethylene (n,n'-bpe, where n = n' = 3 or 4) to undergo an intermolecular [2 + 2] photodimerization in the solid state. The components of cocrystals [(1,4-bd)·(4,4'-bpe)]n and [(1,4-bd)·(3,3'-bpe)]n form 1D hydrogen-bonded polymers with n,n'-bpe assembled as infinite parallel stacks. The alkenes undergo [2 + 2] photocycloadditions to form rctt-tetrakis (n-pyridyl) cyclobutane (where n = 3 or 4). We demonstrate that the reactive solid involving 4,4'-bpe exhibits supramolecular catalysis.
Keywords: [2 + 2] photocycloaddition; divergent template; solid state; supramolecular catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Campillo-Alvarado G., Brannan A.D., Swenson D.C., MacGillivray L.R. Exploiting the hydrogen-bonding capacity of organoboronic acids to direct covalent bond formation in the solid state: Templation and catalysis of the [2 + 2] photodimerization. Org. Lett. 2018;20:5490–5492. doi: 10.1021/acs.orglett.8b02439. - DOI - PubMed
-
- Toda F., Tanaka K., Sekikawa A. Host-guest complex formation by a solid-solid reaction. J. Chem. Soc. Chem. Commun. 1987:279–280. doi: 10.1039/C39870000279. - DOI
-
- Toda F. Reaction control by a host-guest complexation method. J. Inclus. Phenom. Mol. Recognit. Chem. 1989;7:247–256. doi: 10.1007/BF01060725. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

