Systemic acquired resistance networks amplify airborne defense cues
- PMID: 31444353
- PMCID: PMC6707303
- DOI: 10.1038/s41467-019-11798-2
Systemic acquired resistance networks amplify airborne defense cues
Abstract
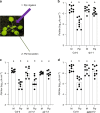
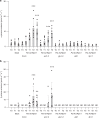
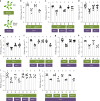
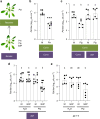
Salicylic acid (SA)-mediated innate immune responses are activated in plants perceiving volatile monoterpenes. Here, we show that monoterpene-associated responses are propagated in feed-forward loops involving the systemic acquired resistance (SAR) signaling components pipecolic acid, glycerol-3-phosphate, and LEGUME LECTIN-LIKE PROTEIN1 (LLP1). In this cascade, LLP1 forms a key regulatory unit in both within-plant and between-plant propagation of immunity. The data integrate molecular components of SAR into systemic signaling networks that are separate from conventional, SA-associated innate immune mechanisms. These networks are central to plant-to-plant propagation of immunity, potentially raising SAR to the population level. In this process, monoterpenes act as microbe-inducible plant volatiles, which as part of plant-derived volatile blends have the potential to promote the generation of a wave of innate immune signaling within canopies or plant stands. Hence, plant-to-plant propagation of SAR holds significant potential to fortify future durable crop protection strategies following a single volatile trigger.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

