Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality
- PMID: 31444469
- PMCID: PMC6796935
- DOI: 10.1038/s41422-019-0214-z
Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality
Abstract
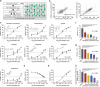
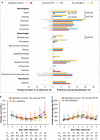
Severe fever with thrombocytopenia syndrome (SFTS), an emerging tick-borne infectious disease caused by a novel phlebovirus (SFTS virus, SFTSV), was listed among the top 10 priority infectious diseases by the World Health Organization due to its high fatality of 12%-50% and possibility of pandemic transmission. Currently, effective anti-SFTSV intervention remains unavailable. Here, by screening a library of FDA-approved drugs, we found that benidipine hydrochloride, a calcium channel blocker (CCB), inhibited SFTSV replication in vitro. Benidipine hydrochloride was revealed to inhibit virus infection through impairing virus internalization and genome replication. Further experiments showed that a broad panel of CCBs, including nifedipine, inhibited SFTSV infection. The anti-SFTSV effect of these two CCBs was further analyzed in a humanized mouse model in which CCB treatment resulted in reduced viral load and decreased fatality rate. Importantly, by performing a retrospective clinical investigation on a large cohort of 2087 SFTS patients, we revealed that nifedipine administration enhanced virus clearance, improved clinical recovery, and remarkably reduced the case fatality rate by >5-fold. These findings are highly valuable for developing potential host-oriented therapeutics for SFTS and other lethal acute viral infections known to be inhibited by CCBs in vitro.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 31770188/National Natural Science Foundation of China (National Science Foundation of China)
- 81472005/National Natural Science Foundation of China (National Science Foundation of China)
- 81722041/National Natural Science Foundation of China (National Science Foundation of China)
- 31500144/National Natural Science Foundation of China (National Science Foundation of China)
- 81825019/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

