Protozoan persister-like cells and drug treatment failure
- PMID: 31444481
- PMCID: PMC7024564
- DOI: 10.1038/s41579-019-0238-x
Protozoan persister-like cells and drug treatment failure
Abstract
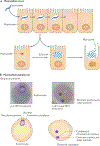
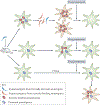
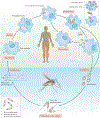
Antimicrobial treatment failure threatens our ability to control infections. In addition to antimicrobial resistance, treatment failures are increasingly understood to derive from cells that survive drug treatment without selection of genetically heritable mutations. Parasitic protozoa, such as Plasmodium species that cause malaria, Toxoplasma gondii and kinetoplastid protozoa, including Trypanosoma cruzi and Leishmania spp., cause millions of deaths globally. These organisms can evolve drug resistance and they also exhibit phenotypic diversity, including the formation of quiescent or dormant forms that contribute to the establishment of long-term infections that are refractory to drug treatment, which we refer to as 'persister-like cells'. In this Review, we discuss protozoan persister-like cells that have been linked to persistent infections and discuss their impact on therapeutic outcomes following drug treatment.
Conflict of interest statement
There is
Figures


References
-
- O’Neill J Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. http://amr-review.org/Publications (2014).
-
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO & Piddock LJ Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol 13, 42–51 (2015). - PubMed
-
- Bigger JW The bactericidal action of penicillin on Staphylococcus pyogenes. Irish J. Med. Sci 19, 585–595 (1944).
-
- Lewis K Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol 5, 48–56 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

