Dissecting the genetic basis of focal cortical dysplasia: a large cohort study
- PMID: 31444548
- PMCID: PMC6851393
- DOI: 10.1007/s00401-019-02061-5
Dissecting the genetic basis of focal cortical dysplasia: a large cohort study
Abstract
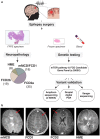
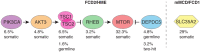
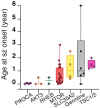
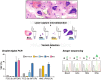
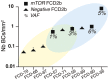
Genetic malformations of cortical development (MCDs), such as mild MCDs (mMCD), focal cortical dysplasia (FCD), and hemimegalencephaly (HME), are major causes of severe pediatric refractory epilepsies subjected to neurosurgery. FCD2 are characterized by neuropathological hallmarks that include enlarged dysmorphic neurons (DNs) and balloon cells (BCs). Here, we provide a comprehensive assessment of the contribution of germline and somatic variants in a large cohort of surgical MCD cases. We enrolled in a monocentric study 80 children with drug-resistant epilepsy and a postsurgical neuropathological diagnosis of mMCD, FCD1, FCD2, or HME. We performed targeted gene sequencing ( ≥ 2000X read depth) on matched blood-brain samples to search for low-allele frequency variants in mTOR pathway and FCD genes. We were able to elucidate 29% of mMCD/FCD1 patients and 63% of FCD2/HME patients. Somatic loss-of-function variants in the N-glycosylation pathway-associated SLC35A2 gene were found in mMCD/FCD1 cases. Somatic gain-of-function variants in MTOR and its activators (AKT3, PIK3CA, RHEB), as well as germline, somatic and two-hit loss-of-function variants in its repressors (DEPDC5, TSC1, TSC2) were found exclusively in FCD2/HME cases. We show that panel-negative FCD2 cases display strong pS6-immunostaining, stressing that all FCD2 are mTORopathies. Analysis of microdissected cells demonstrated that DNs and BCs carry the pathogenic variants. We further observed a correlation between the density of pathological cells and the variant-detection likelihood. Single-cell microdissection followed by sequencing of enriched pools of DNs unveiled a somatic second-hit loss-of-heterozygosity in a DEPDC5 germline case. In conclusion, this study indicates that mMCD/FCD1 and FCD2/HME are two distinct genetic entities: while all FCD2/HME are mosaic mTORopathies, mMCD/FCD1 are not caused by mTOR-pathway-hyperactivating variants, and ~ 30% of the cases are related to glycosylation defects. We provide a framework for efficient genetic testing in FCD/HME, linking neuropathology to genetic findings and emphasizing the usefulness of molecular evaluation in the pediatric epileptic neurosurgical population.
Keywords: Brain mosaicism; Epilepsy-associated focal cortical dysplasia; Neurogenetics; Somatic variant; mTOR pathway.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Baulac S, Ishida S, Marsan E, Miquel C, Biraben A, Nguyen DK, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77:675–683. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

