Tmem2 restricts atrioventricular canal differentiation by regulating degradation of hyaluronic acid
- PMID: 31444829
- PMCID: PMC8582300
- DOI: 10.1002/dvdy.106
Tmem2 restricts atrioventricular canal differentiation by regulating degradation of hyaluronic acid
Abstract
Background: Atrioventricular valve development relies upon the precisely defined dimensions of the atrioventricular canal (AVC). Current models suggest that Wnt signaling plays an important role atop a pathway that promotes AVC development. The factors that confine AVC differentiation to the appropriate location, however, are less well understood.
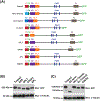
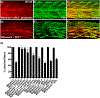
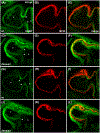
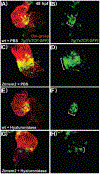
Results: Transmembrane protein 2 (Tmem2) is a key player in restricting AVC differentiation: in zebrafish, tmem2 mutants display an expansion of AVC characteristics, but the molecular mechanism of Tmem2 function in this context remains unclear. Through structure-function analysis, we demonstrate that the extracellular portion of Tmem2 is crucial for its role in restricting AVC boundaries. Importantly, the Tmem2 ectodomain contains regions implicated in the depolymerization of hyaluronic acid (HA). We find that tmem2 mutant hearts exhibit excess HA deposition alongside broadened distribution of Wnt signaling. Moreover, addition of ectopic hyaluronidase can restore the restriction of AVC differentiation in tmem2 mutants. Finally, we show that alteration of a residue important for HA depolymerization impairs the efficacy of Tmem2 function during AVC development.
Conclusions: Taken together, our data support a model in which HA degradation, regulated by Tmem2, limits the distribution of Wnt signaling and thereby confines the differentiation of the AVC.
Keywords: Cemip2; Wnt signaling; cardiac fusion; hyaluronidase; valve formation.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
COMPETING INTERESTS
No competing interests declared.
Figures






Similar articles
-
Genomic and physiological analyses of the zebrafish atrioventricular canal reveal molecular building blocks of the secondary pacemaker region.Cell Mol Life Sci. 2021 Oct;78(19-20):6669-6687. doi: 10.1007/s00018-021-03939-y. Epub 2021 Sep 23. Cell Mol Life Sci. 2021. PMID: 34557935 Free PMC article.
-
Tmem2 Regulates Embryonic Vegf Signaling by Controlling Hyaluronic Acid Turnover.Dev Cell. 2017 Jan 23;40(2):123-136. doi: 10.1016/j.devcel.2016.12.017. Dev Cell. 2017. PMID: 28118600
-
The novel transmembrane protein Tmem2 is essential for coordination of myocardial and endocardial morphogenesis.Development. 2011 Oct;138(19):4199-205. doi: 10.1242/dev.064261. Development. 2011. PMID: 21896630 Free PMC article.
-
New molecules indispensable for hyaluronan degradation, HYBID (CEMIP/KIAA1199) and TMEM2 (CEMIP2): Differential roles in physiological and pathological non-neoplastic conditions.Proc Jpn Acad Ser B Phys Biol Sci. 2025;101(6):317-338. doi: 10.2183/pjab.101.021. Proc Jpn Acad Ser B Phys Biol Sci. 2025. PMID: 40500182 Free PMC article. Review.
-
TMEM2: A missing link in hyaluronan catabolism identified?Matrix Biol. 2019 May;78-79:139-146. doi: 10.1016/j.matbio.2018.03.020. Epub 2018 Mar 27. Matrix Biol. 2019. PMID: 29601864 Free PMC article. Review.
Cited by
-
Structure of the transmembrane protein 2 (TMEM2) ectodomain and its apparent lack of hyaluronidase activity.Wellcome Open Res. 2023 May 2;8:76. doi: 10.12688/wellcomeopenres.18937.2. eCollection 2023. Wellcome Open Res. 2023. PMID: 37234743 Free PMC article.
-
Genetic Deficiencies of Hyaluronan Degradation.Cells. 2024 Jul 16;13(14):1203. doi: 10.3390/cells13141203. Cells. 2024. PMID: 39056785 Free PMC article. Review.
-
Epithelial-Mesenchymal Transition: Role in Cancer Progression and the Perspectives of Antitumor Treatment.Acta Naturae. 2020 Jul-Sep;12(3):4-23. doi: 10.32607/actanaturae.11010. Acta Naturae. 2020. PMID: 33173593 Free PMC article.
-
Mechanical forces remodel the cardiac extracellular matrix during zebrafish development.Development. 2024 Jul 1;151(13):dev202310. doi: 10.1242/dev.202310. Epub 2024 Jul 10. Development. 2024. PMID: 38984541 Free PMC article.
-
Zebrafish Models in Therapeutic Research of Cardiac Conduction Disease.Front Cell Dev Biol. 2021 Aug 4;9:731402. doi: 10.3389/fcell.2021.731402. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34422842 Free PMC article. Review.
References
-
- Abe S, Usami S, Nakamura Y. 2003. Mutations in the gene encoding KIAA1199 protein, an inner-ear protein expressed in Deiters’ cells and the fibrocytes, as the cause of nonsyndromic hearing loss. J Hum Genet 48:564–570. - PubMed
-
- Alexander J, Rothenberg M, Henry GL, Stainier DY. 1999. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol 215:343–357. - PubMed
-
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr., Kubalak S, Klewer SE, McDonald JA. 2000. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 106:349–360. - PMC - PubMed
-
- De Angelis JE, Lagendijk AK, Chen H, Tromp A, Bower NI, Tunny KA, Brooks AJ, Bakkers J, Francois M, Yap AS, Simons C, Wicking C, Hogan BM, Smith KA. 2017. Tmem2 Regulates Embryonic Vegf Signaling by Controlling Hyaluronic Acid Turnover. Dev Cell 40:123–136. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

