Common variable immunodeficiency-associated endotoxemia promotes early commitment to the T follicular lineage
- PMID: 31445098
- PMCID: PMC6900457
- DOI: 10.1016/j.jaci.2019.08.007
Common variable immunodeficiency-associated endotoxemia promotes early commitment to the T follicular lineage
Abstract
Background: Although chiefly a B-lymphocyte disorder, several research groups have identified common variable immunodeficiency (CVID) subjects with numeric and/or functional TH cell alterations. The causes, interrelationships, and consequences of CVID-associated CD4+ T-cell derangements to hypogammaglobulinemia, autoantibody production, or both remain unclear.
Objective: We sought to determine how circulating CD4+ T cells are altered in CVID subjects with autoimmune cytopenias (AICs; CVID+AIC) and the causes of these derangements.
Methods: Using hypothesis-generating, high-dimensional single-cell analyses, we created comprehensive phenotypic maps of circulating CD4+ T cells. Differences between subject groups were confirmed in a large and genetically diverse cohort of CVID subjects (n = 69) by using flow cytometry, transcriptional profiling, multiplex cytokine/chemokine detection, and a suite of in vitro functional assays measuring naive T-cell differentiation, B-cell/T-cell cocultures, and regulatory T-cell suppression.
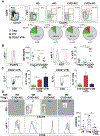
Results: Although CD4+ TH cell profiles from healthy donors and CVID subjects without AICs were virtually indistinguishable, T cells from CVID+AIC subjects exhibited follicular features as early as thymic egress. Follicular skewing correlated with IgA deficiency-associated endotoxemia and endotoxin-induced expression of activin A and inducible T-cell costimulator ligand. The resulting enlarged circulating follicular helper T-cell population from CVID+AIC subjects provided efficient help to receptive healthy donor B cells but not unresponsive CVID B cells. Despite this, circulating follicular helper T cells from CVID+AIC subjects exhibited aberrant transcriptional profiles and altered chemokine/cytokine receptor expression patterns that interfered with regulatory T-cell suppression assays and were associated with autoantibody production.
Conclusions: Endotoxemia is associated with early commitment to the follicular T-cell lineage in IgA-deficient CVID subjects, particularly those with AICs.
Keywords: Common variable immunodeficiency; activin A; autoimmune cytopenias; endotoxin; follicular helper T cell; recent thymic emigrant; regulatory T cell; time-of-flight cytometry.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


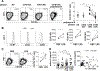
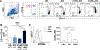

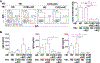
References
-
- Romberg NNY, Cunningham-Rundles C, Meffre E. Response: common variable immunodeficiency patients with increased CD21−/lo B cells suffer from altered receptor editing and defective central B-cell tolerance. Blood 2011.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

