A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells
- PMID: 31446050
- PMCID: PMC6801041
- DOI: 10.1016/j.actbio.2019.08.031
A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells
Abstract
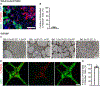
Differentiation of stem cells into functional replacement cells and tissues is a major goal of the regenerative medicine field. However, one limitation has been organization of differentiated cells into multi-cellular, three-dimensional assemblies. The islets of Langerhans contain many endocrine and non-endocrine cell types, such as insulin-producing β cells and endothelial cells. Despite the potential importance of endothelial cells to islet function, facilitating interactions between endothelial cells and islet endocrine cell types already differentiated from human embryonic stem cells has been difficult in vitro. We have developed a strategy of assembling human embryonic stem cell-derived islet cells with endothelial cells into three-dimensional aggregates on a hydrogel. The resulting islet organoids express β cell and other endocrine markers and are functional, capable of undergoing glucose-stimulated insulin secretion. This assembly was not observed on traditional tissue culture plastic and in aggregates generated in suspension culture, highlighting how physical culture conditions greatly influence the interactions among these cell types. These results provide a platform for evaluating the effects of the islet tissue microenvironment on human embryonic stem cell-derived β cells and other islet endocrine cells to develop tissue engineered islets. STATEMENT OF SIGNIFICANCE: Differentiation of insulin-producing cells and tissues from human pluripotent stem cells is being investigated for diabetes cell replacement therapies. Despite successes generating β cells, the cell type responsible for glucose-stimulated insulin secretion within the islets of Langerhans found in the pancreas, successful assembly with other non-endocrine cell types, particularly endothelial cells, has been technically challenging. The present study provides a platform for the assembly of endothelial cells with SC-β and other endocrine cells, producing islet organoids that are functional and express β cell markers, that can be used to study the islet microenvironment and islet tissue engineering.
Keywords: Biomaterials; Diabetes; Organoids; Stem cells; Tissue engineering.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

