Neutral Lipopolyplexes for In Vivo Delivery of Conventional and Replicative RNA Vaccine
- PMID: 31446119
- PMCID: PMC6716064
- DOI: 10.1016/j.omtn.2019.07.014
Neutral Lipopolyplexes for In Vivo Delivery of Conventional and Replicative RNA Vaccine
Abstract
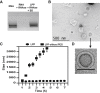
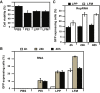
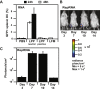
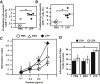
Nucleic acid vaccination relies on injecting DNA or RNA coding antigen(s) to induce a protective immune response. RNA vaccination is being increasingly used in preclinical and clinical studies. However, few delivery systems have been reported for in vivo delivery of RNA of different sizes. Using a tripartite formulation with RNA, cationic polymer, and anionic liposomes, we were able to encapsulate RNA into neutral lipopolyplexes (LPPs). LPPs were stable in vitro and successfully delivered conventional RNA and replicative RNA to dendritic cells in cellulo. Their injection led to reporter gene expression in mice. Finally, administration of LPP-Replicon RNA (RepRNA) led to an adaptive immune response against the antigen coded by the RepRNA. Accordingly, LPPs may represent a universal formulation for RNA delivery.
Keywords: mRNA delivery; self-amplifying RNA; splenic dendritic cells; targeting.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
-
- Perche F., Uchida S., Akiba H., Lin C.-Y., Ikegami M., Dirisala A., Nakashima T., Itaka K., Tsumoto K., Kataoka K. Improved brain expression of anti-amyloid β scfv by complexation of mRNA including a secretion sequence with PEG-based block catiomer. Curr. Alzheimer Res. 2017;14:295–302. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

