A System for Analog Control of Cell Culture Dynamics to Reveal Capabilities of Signaling Networks
- PMID: 31446223
- PMCID: PMC6713801
- DOI: 10.1016/j.isci.2019.08.010
A System for Analog Control of Cell Culture Dynamics to Reveal Capabilities of Signaling Networks
Abstract
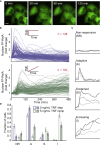
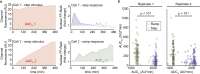
Cellular microenvironments are dynamic. When exposed to extracellular cues, such as changing concentrations of inflammatory cytokines, cells activate signaling networks that mediate fate decisions. Exploring responses broadly to time-varying microenvironments is essential to understand the information transmission capabilities of signaling networks and how dynamic milieus influence cell fate decisions. Here, we present a gravity-driven cell culture and demonstrate that the system accurately produces user-defined concentration profiles for one or more dynamic stimuli. As proof of principle, we monitor nuclear factor-κB activation in single cells exposed to dynamic cytokine stimulation and reveal context-dependent sensitivity and uncharacterized single-cell response classes distinct from persistent stimulation. Using computational modeling, we find that cell-to-cell variability in feedback rates within the signaling network contributes to different response classes. Models are validated using inhibitors to predictably modulate response classes in live cells exposed to dynamic stimuli. These hidden capabilities, uncovered through dynamic stimulation, provide opportunities to discover and manipulate signaling mechanisms.
Keywords: Biological Science Instrumentation; Complex Systems; Immunology; Mathematical Biosciences.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

