A Brain-Targeted Orally Available ROCK2 Inhibitor Benefits Mild and Aggressive Cavernous Angioma Disease
- PMID: 31446620
- PMCID: PMC7036327
- DOI: 10.1007/s12975-019-00725-8
A Brain-Targeted Orally Available ROCK2 Inhibitor Benefits Mild and Aggressive Cavernous Angioma Disease
Abstract
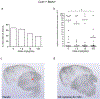
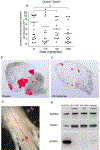
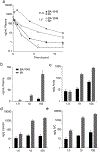
Cavernous angioma (CA) is a vascular pathology caused by loss of function in one of the 3 CA genes (CCM1, CCM2, and CCM3) that result in rho kinase (ROCK) activation. We investigated a novel ROCK2 selective inhibitor for the ability to reduce brain lesion formation, growth, and maturation. We used genetic methods to explore the use of a ROCK2-selective kinase inhibitor to reduce growth and hemorrhage of CAs. The role of ROCK2 in CA was investigated by crossing Rock1 or Rock2 hemizygous mice with Ccm1 or Ccm3 hemizygous mice, and we found reduced lesions in the Rock2 hemizygous mice. A ROCK2-selective inhibitor, BA-1049 was used to investigate efficacy in reducing CA lesions after oral administration to Ccm1+/- and Ccm3+/- mice that were bred into a mutator background. After assessing the dose range effective to target brain endothelial cells in an ischemic brain model, Ccm1+/- and Ccm3+/- transgenic mice were treated for 3 (Ccm3+/-) or 4 months (Ccm1+/-), concurrently, randomized to receive one of three doses of BA-1049 in drinking water, or placebo. Lesion volumes were assessed by micro-computed tomography. BA-1049 reduced activation of ROCK2 in Ccm3+/-Trp53-/- lesions. Ccm1+/-Msh2-/- (n=68) and Ccm3+/-Trp53-/- (n=71) mice treated with BA-1049 or placebo showed a significant dose-dependent reduction in lesion volume after treatment with BA-1049, and a reduction in hemorrhage (iron deposition) near lesions at all doses. These translational studies show that BA-1049 is a promising therapeutic agent for the treatment of CA, a disease with no current treatment except surgical removal of the brain lesions.
Keywords: Cerebral cavernous malformation; Hemangioma; Rho kinase; Therapeutics; Vascular permeability.
Conflict of interest statement
Competing interests
Dr. McKerracher is CEO of BioAxone, holds an ownership interest in the company and has a significant competing interest. Drs. Matthew Abbinanti, Ken Rosen and Joerg Ruschel were employees of BioAxone and have a modest conflict of interest. Drs. Doug Marchuk and Issam Awad were recipients of sponsored research through the SBIR grant to BioAxone. The other authors declare no conflict of interest.
Figures

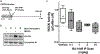


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

