Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection
- PMID: 31446622
- PMCID: PMC6708604
- DOI: 10.1002/14651858.CD009417.pub2
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection
Update in
-
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD009417. doi: 10.1002/14651858.CD009417.pub3. Cochrane Database Syst Rev. 2023. PMID: 37870128 Free PMC article.
Abstract
Background: Millions of children are hospitalised due to respiratory syncytial virus (RSV) infection every year. Treatment is supportive, and current therapies (e.g. inhaled bronchodilators, epinephrine, nebulised hypertonic saline, and corticosteroids) are ineffective or have limited effect. Respiratory syncytial virus immunoglobulin is sometimes used prophylactically to prevent hospital admission from RSV-related illness. It may be considered for the treatment of established severe RSV infection or for treatment in an immunocompromised host, although it is not licenced for this purpose. It is unclear whether immunoglobulins improve outcomes when used as a treatment for established RSV infection in infants and young children admitted to hospital. OBJECTIVES: To assess the effects of immunoglobulins for the treatment of RSV-proven lower respiratory tract infections in children aged up to three years, admitted to hospital. SEARCH METHODS: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, Ovid MEDLINE, Embase, CINAHL, and Web of Science (from inception to 6 November 2018) with no restrictions. We searched two trial registries for ongoing trials (to 30 March 2018) and checked the reference lists of reviews and included articles for additional studies.
Selection criteria: Randomised controlled trials comparing immunoglobulins with placebo in hospitalised infants and children aged up to three years with laboratory-diagnosed RSV lower respiratory tract infection.
Data collection and analysis: Two review authors independently selected trials, assessed risk of bias, and extracted data. We assessed evidence quality using GRADE.
Main results: We included seven trials involving 486 infants and children aged up to three years. The immunoglobulin preparations used in these trials included anti-RSV immunoglobulin and the monoclonal antibody preparations palivizumab and motavizumab. We assessed the primary outcomes of mortality, length of hospital stay, and adverse events as providing low- or very low-certainty evidence due to risk of bias and imprecision. All trials were conducted at sites in high-income countries (USA, Chile, New Zealand, Australia), with two studies including a site in a middle-income country (Panama). Five of the seven studies were "supported" or "sponsored" by the trial drug manufacturers. We found no evidence of a difference between immunoglobulins and placebo for mortality (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.14 to 5.27; 3 trials; 196 children; 4 deaths; 2 deaths amongst 98 children receiving immunoglobulins, and 2 deaths amongst 98 children receiving placebo. One additional death occurred in a fourth trial, however, the study group of the child was not known and the data were not included in the analysis; very low-certainty evidence), and length of hospitalisation (mean difference -0.70, 95% CI -1.83 to 0.42; 5 trials; 324 children; low-certainty evidence). There was no evidence of a difference between immunoglobulins and placebo in adverse events of any severity or seriousness (reported in five trials) or serious adverse events (four trials) (RR for any severity 1.18, 95% CI 0.78 to 1.78; 340 children; low-certainty evidence, and for serious adverse events 1.08, 95% CI 0.65 to 1.79; 238 children; low-certainty evidence).We found no evidence of a significant difference between immunoglobulins and placebo for any of our secondary outcomes. We identified one ongoing trial.
Authors' conclusions: We found insufficient evidence of a difference between immunoglobulins and placebo for any review outcomes. We assessed the evidence for the effects of immunoglobulins when used as a treatment for RSV lower respiratory tract infection in hospitalised infants and young children as of low or very low certainty due to risk of bias and imprecision. We are uncertain of the effects of immunoglobulins on these outcomes, and the true effect may be substantially different from the effects reported in this review. All trials were conducted in high-income countries, and data from populations in which the rate of death from RSV infection is higher are lacking.
Conflict of interest statement
Sharon L Sanders: none known Sushil Agwan: none known Mohamed Hassan: none known Mieke L van Driel: none known Chris B Del Mar: none known
Figures
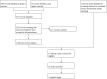
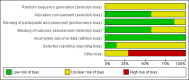




References
References to studies included in this review
Hemming 1987 {published data only}
Lagos 2009 {published data only}
-
- Lagos R, DeVincenzo JP, Muñoz A, Hultquist M, Suzich JA, Connor EM, et al. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)–specific humanized monoclonal antibody, when administered to RSV‐infected children. Pediatric Infectious Disease Journal 2009;28(9):835‐7. [DOI: 10.1097/INF.0b013e3181a165e4] - DOI - PubMed
Malley 1998 {published data only}
-
- Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner C, Gruber WC, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. Journal of Infectious Diseases 1998;178(6):1555‐61. - PubMed
Ramilo 2014 {published data only}
-
- Ramilo O, Lagos R, Sáez‐Llorens X, Suzich J, Wang CK, Jensen KM, et al. Motavizumab Study Group. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatric Infectious Disease Journal 2014;33(7):703‐9. [DOI: 10.1097/INF.0000000000000240] - DOI - PubMed
Rodriguez 1997a {published data only}
-
- Rodriguez WJ, Gruber WC, Welliver RC, Groothuis JR, Simoes EAF, Meissner HC, et al. Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections. Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics 1997;99(3):454‐61. - PubMed
Rodriguez 1997b {published data only}
-
- Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EAF, Rosas AJ, Lepow M, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 1997;100(6):937‐42. - PubMed
Sáez‐Llorens 2004 {published data only}
-
- Sáez‐Llorens X, Moreno MT, Ramilo O, Sánchez PJ, Top FH Jr, Connor EM, MEDI‐493 Study Group. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatric Infectious Disease Journal 2004;23(8):707‐12. [DOI: 10.1097/01.inf.0000133165.85909.08] - DOI - PubMed
References to studies excluded from this review
AAP 1998 {published data only}
-
- Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV‐IGIV. American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. Pediatrics 1998;102(5):1211‐6. - PubMed
Faber 2008 {published data only}
-
- Faber TE, Kimpen JL, Bont LJ. Respiratory syncytial virus bronchiolitis: prevention and treatment. Expert Opinion on Pharmacotherapy 2008;9(14):2451‐8. - PubMed
Feltes 2011 {published data only}
-
- Feltes TF, Sondheimer HM, Tulloh RM, Harris BS, Jensen KM, Losonsky GA, et al. Motavizumab Cardiac Study Group. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatric Research 2011;70(2):186‐91. - PubMed
Fernández 2010 {published data only}
-
- Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, et al. Motavizumab Study Group. A phase 2, randomized, double‐blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatrics 2010;10:38. [DOI: 10.1186/1471-2431-10-38] - DOI - PMC - PubMed
Givner 1999 {published data only}
-
- Givner LB. Monoclonal antibodies against respiratory syncytial virus. Pediatric Infectious Disease Journal 1999;18(6):541‐2. - PubMed
Halsey 1997 {published data only}
-
- Halsey NA, Abramson JS, Chesney PJ, Fisher MC, Gerber MA, Gromisch DS, et al. Respiratory syncytial virus immune globulin intravenous: indications for use. Pediatrics 1997;99(4):645‐50. - PubMed
Harkensee 2006 {published data only}
-
- Harkensee C, Brodlie M, Embleton ND, Mckean M. Passive immunisation of preterm infants with palivizumab against RSV infection. Journal of Infection 2006;52(1):2‐8. - PubMed
Helmink 2016 {published data only}
Hu 2010 {published data only}
-
- Hu J, Robinson JL. Treatment of respiratory syncytial virus with palivizumab: a systematic review. World Journal of Pediatrics 2010;6(4):296‐300. - PubMed
Wegzyn 2014 {published data only}
References to ongoing studies
NCT02442427 {published data only}
-
- NCT02442427. Palivizumab therapy for RSV‐bronchiolitis. clinicaltrials.gov/ct2/show/NCT02442427 (first received 13 May 2015).
Additional references
American Academy of Pediatrics 2014
-
- American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134(2):415‐520. - PubMed
American Academy of Pediatrics 2015
-
- American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS editor(s). Red Book: 2015 Report of the Committee on Infectious Diseases. 30th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 2015:667‐76.
American Academy of Pediatrics 2018
-
- American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS editor(s). Red Book: 2018 Report of the Committee on Infectious Diseases. 1st Edition. Itasca, IL: American Academy of Pediatrics, 2018:682‐92.
Broadbent 2015
-
- Broadbent L, Groves H, Shields MD, Power UF. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza and Other Respiratory Viruses 2015;9(4):169‐78. - PMC - PubMed
Feltes 2003
-
- Feltes TF, Cablka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Journal of Pediatrics 2003;143(4):532‐40. - PubMed
Fernandes 2013
Gadomski 2014
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime).
Greenough 2001
Griffiths 2017
Groothuis 1993
-
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high‐risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. New England Journal of Medicine 1993;329(21):1524‐30. - PubMed
Hartling 2011
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
Jolles 2005
Langley 1997
-
- Langley JM, Wang EE, Law BJ, Stephens D, Boucher FD, Dobson S, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. Journal of Pediatrics 1997;131:113‐7. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mayo Clinic 2017
-
- Mayo Clinic. Respiratory syncytial virus. www.mayoclinic.org/diseases‐conditions/respiratory‐syncytial‐virus/sympt... (accessed prior to 9 January 2019).
Mejías 2005
Nair 2010
Oray‐Schrom 2003
-
- Oray‐Schrom P, Phoenix C, Martin D, Amoateng‐Adjepong Y. Sepsis workup in febrile infants 0‐90 days of age with respiratory syncytial virus infection. Pediatric Emergency Care 2003;19(5):314‐9. - PubMed
Paramore 2004
-
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus‐related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275‐84. - PubMed
PREVENT 1997
-
- PREVENT Study Group. Reduction of RSV hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997;99(1):93‐9. - PubMed
Ralston 2009
-
- Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. Journal of Pediatrics 2009;155(5):728‐33. - PubMed
Resch 2017
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roche 2003
-
- Roche P, Lambert S, Spencer J. Surveillance of viral pathogens in Australia: respiratory syncytial virus. Communicable Diseases Intelligence 2003;27(1):117‐22. - PubMed
Rodriguez 1997
-
- Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EA, Rosas AJ, Lepow M, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 2007;100(6):937‐42. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shi 2017
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390(10098):946‐58. - PMC - PubMed
Synagis 2017
-
- Synagis. Highlights of prescribing information. www.azpicentral.com/synagis/synagis.pdf (accessed prior to 5 December 2018).
Turner 2014
Wang 2011
Zhang 2017
References to other published versions of this review
Fuller 2004
Fuller 2006
Kantharajah 2011
-
- Kantharajah M, Zaman FD, Samra D, Gunturu MN, Mar CB, Driel ML. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009417] - DOI
Tan 1998
-
- Tan D, Wang E, Ohlsson A. Immunoglobulin for treatment of respiratory syncytial virus. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000981] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

