Smoking cessation interventions for potential use in the lung cancer screening setting: A systematic review and meta-analysis
- PMID: 31446996
- PMCID: PMC6739236
- DOI: 10.1016/j.lungcan.2019.06.024
Smoking cessation interventions for potential use in the lung cancer screening setting: A systematic review and meta-analysis
Abstract
Objectives: Current guidelines recommend delivery of smoking cessation interventions with lung cancer screening (LCS). Unfortunately, there are limited data to guide clinicians and policy-makers in choosing cessation interventions in this setting. Several trials are underway to fill this evidence gap, but results are not expected for several years.
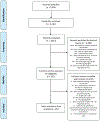
Methods and materials: We conducted a systematic review and meta-analysis of current literature on the efficacy of smoking cessation interventions among populations eligible for LCS. We searched PubMed, Medline, and PsycINFO for randomized controlled trials of smoking cessation interventions published from 2010-2017. Trials were eligible for inclusion if they sampled individuals likely to be eligible for LCS based on age and smoking history, had sample sizes >100, follow-up of 6- or 12-months, and were based in North America, Western Europe, Australia, or New Zealand.
Results: Three investigators independently screened 3,813 abstracts and identified 332 for full-text review. Of these, 85 trials were included and grouped into categories based on the primary intervention: electronic/web-based, in-person counseling, pharmacotherapy, and telephone counseling. At 6-month follow-up, electronic/web-based (odds ratio [OR] 1.14, 95% CI 1.03-1.25), in-person counseling (OR 1.46, 95% CI 1.25-1.70), and pharmacotherapy (OR 1.53, 95% CI 1.33-1.77) interventions significantly increased the odds of abstinence. Telephone counseling increased the odds but did not reach statistical significance (OR 1.21, 95% CI 0.98-1.50). At 12-months, in-person counseling (OR 1.28 95% CI 1.10-1.50) and pharmacotherapy (OR 1.46, 95% CI 1.17-1.84) remained efficacious, although the decrement in efficacy was of similar magnitude across all intervention categories.
Conclusions: Several categories of cessation interventions are promising for implementation in the LCS setting.
Keywords: Lung cancer screening; Meta-analysis; Smoking cessation.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of Interest:
None declared.
Figures






Comment in
-
Novelties in Lung Cancer Screening.Am J Respir Crit Care Med. 2021 Sep 1;204(5):596-598. doi: 10.1164/rccm.202012-4505RR. Am J Respir Crit Care Med. 2021. PMID: 34213386 No abstract available.
References
-
- De Koning HJ, Van der Aalst CM, ten Haaf K, Oudkerk M. Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. . IASLC WCLC 2018. Toronto; 2018.
-
- U.S.Preventive Services Task Force. Final Recommendation Statement: Lung Cancer: Screening. October 2014. http://www.uspreventiveservicestaskforce.org/Page/Document/Recommendatio... Accessed December 18, 2017.
-
- National Comprehensive Cancer Network. NCCN Guidelines for Patients: Lung Cancer Screening. 2017. https://www.nccn.org/patients/guidelines/lung_screening/files/assets/bas... Accessed December 18, 2017.
-
- Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56(1):125–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

