Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer
- PMID: 31447322
- PMCID: PMC7616630
- DOI: 10.1016/j.cmet.2019.07.015
Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer
Abstract
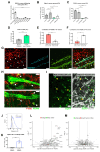
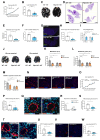
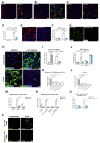
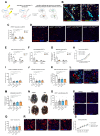
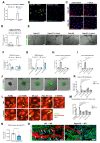
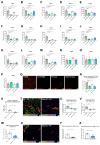
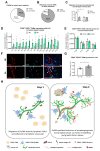
Among mammary tumor-infiltrating immune cells, the highest expression of podoplanin (PDPN) is found in a subset of tumor-associated macrophages (TAMs). We hereby demonstrate that PDPN is involved in the attachment of this TAM subset to lymphatic endothelial cells (LECs). Mechanistically, the binding of PDPN to LEC-derived galectin 8 (GAL8) in a glycosylation-dependent manner promotes the activation of pro-migratory integrin β1. When proximal to lymphatics, PDPN-expressing macrophages (PoEMs) stimulate local matrix remodeling and promote vessel growth and lymphoinvasion. Anti-integrin β1 blockade, macrophage-specific Pdpn knockout, or GAL8 inhibition impairs TAM adhesion to LECs, restraining lymphangiogenesis and reducing lymphatic cancer spread. In breast cancer patients, association of PoEMs with tumor lymphatic vessels correlates with incidences of lymph node and distant organ metastasis.
Keywords: breast cancer; lymph nodes; lymphangiogenesis; lymphoinvasion; metastasis; podoplanin; tumor-associated macrophages.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
References
-
- Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 2012;37:276–289. doi: 10.1016/j.immuni.2012.05.022. - DOI - PMC - PubMed
-
- Barcus CE, O’Leary KA, Brockman JL, Rugowski DE, Liu Y, Garcia N, Yu M, Keely PJ, Eliceiri KW, Schuler LA. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017;19:9. doi: 10.1186/s13058-017-0801-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

