Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake
- PMID: 31447324
- PMCID: PMC6838660
- DOI: 10.1016/j.cmet.2019.07.013
Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake
Abstract
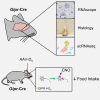
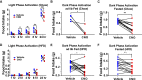
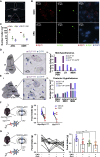
Ambiguity regarding the role of glucose-dependent insulinotropic polypeptide (GIP) in obesity arises from conflicting reports asserting that both GIP receptor (GIPR) agonism and antagonism are effective strategies for inhibiting weight gain. To enable identification and manipulation of Gipr-expressing (Gipr) cells, we created Gipr-Cre knockin mice. As GIPR-agonists have recently been reported to suppress food intake, we aimed to identify central mediators of this effect. Gipr cells were identified in the arcuate, dorsomedial, and paraventricular nuclei of the hypothalamus, as confirmed by RNAscope in mouse and human. Single-cell RNA-seq identified clusters of hypothalamic Gipr cells exhibiting transcriptomic signatures for vascular, glial, and neuronal cells, the latter expressing somatostatin but little pro-opiomelanocortin or agouti-related peptide. Activation of Gq-DREADDs in hypothalamic Gipr cells suppressed food intake in vivo, which was not obviously additive with concomitant GLP1R activation. These data identify hypothalamic GIPR as a target for the regulation of energy balance.
Keywords: food intake; glucose-dependent insulinotropic polypeptide; glucose-dependent insulinotropic polypeptide receptor; hypothalamus.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
F.M.G. is a paid consultant for Kallyope, New York. The Gribble-Reimann lab hosts projects that receive funding from MedImmune/AstraZeneca (F.M.G./F.R.). The F.R./F.M.G. laboratory has recently agreed a collaboration with Lilly on future work in the mechanism of GIPR activation. J.P.-W. joined Novo Nordisk (DK) and E.K.B. joined Absolute Antibody (UK) after completing their contributions to this manuscript while working at IMS. There are no other conflicts of interest to declare.
Figures


References
-
- Boylan M.O., Glazebrook P.A., Tatalovic M., Wolfe M.M. Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. Am. J. Physiol. Endocrinol. Metab. 2015;309:E1008–E1018. - PubMed
-
- Buchan A.M., Polak J.M., Capella C., Solcia E., Pearse A.G. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry. 1978;56:37–44. - PubMed
-
- Buffa R., Polak J.M., Pearse A.G., Solcia E., Grimelius L., Capella C. Identification of the intestinal cell storing gastric inhibitory peptide. Histochemistry. 1975;43:249–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

