Cognitive impairment in heart failure is associated with altered Wnt signaling in the hippocampus
- PMID: 31447429
- PMCID: PMC6738419
- DOI: 10.18632/aging.102150
Cognitive impairment in heart failure is associated with altered Wnt signaling in the hippocampus
Abstract
Age represents the highest risk factor for death due to cardiovascular disease. Heart failure (HF) is the most common cardiovascular disease in elder population and it is associated with cognitive impairment (CI), diminishing learning and memory process affecting life quality and mortality in these patients. In HF, CI has been associated with inadequate O2 supply to the brain; however, an important subset of HF patients displays CI with almost no alteration in cerebral blood flow. Importantly, nothing is known about the pathophysiological mechanisms underpinning CI in HF with no change in brain tissue perfusion. Here, we aimed to study memory performance and learning function in a rodent model of HF that shows no change in blood flow going to the brain. We found that HF rats presented learning impairments and memory loss. In addition, HF rats displayed a decreased level of Wnt/β-catenin signaling downstream elements in the hippocampus, one pathway implicated largely in aging diseases. Taken together, our results suggest that in HF rats CI is associated with dysfunction of the Wnt/β-catenin signaling pathway. The mechanisms involved in the alterations of Wnt/β-catenin signaling in HF and its contribution to the development/maintenance of CI deserves future investigations.
Keywords: Wnt signaling pathway; aging; cognitive impairment; heart failure.
Conflict of interest statement
Figures

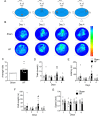
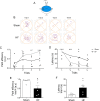
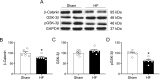
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

