The use of preservatives in dry eye drops
- PMID: 31447543
- PMCID: PMC6682755
- DOI: 10.2147/OPTH.S211611
The use of preservatives in dry eye drops
Abstract
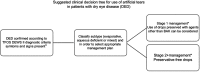
Topical ocular preparations are widely recommended by health care professionals, or chosen by patients, to help manage dry eye disease (DED). The chronic and progressive nature of DED may result in the administration of topical products several times a day, over a period of many years. Given DED is a condition that by definition affects the ocular surface, it is important to understand how the repeated use of eye drops may impact the ocular surface, influence clinical signs, affect symptoms, and impact the overall disease process of dry eye. The component in topical preparations with the greatest potential to adversely affect the ocular surface is the preservative. This paper reviews the literature in relation to the use of preservatives in formulations for dry eye. The ocular effects of benzalkonium chloride (BAK) are summarised and compared to the performance of alternative preservatives and preservative-free formulations. Use of preserved and preservative-free drops in relation to the management of varying stages of DED is discussed.
Keywords: benzalkonium chloride (BAK); dry eye disease; polyquaternium-1 (PQ-1); preservative-free; preservatives.
Conflict of interest statement
Karen Walsh reports personal fees from Alcon, CooperVision and Johnson & Johnson Vision outside of the submitted work. Lyndon Jones reports personal fees from Alcon, CooperVision, Johnson & Johnson Vision, Menicon, Novartis, Ophtecs and Santen outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
-
- Smith JA, Albeitz J, Begley C, et al. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93–107. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

