Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects
- PMID: 31447664
- PMCID: PMC6692637
- DOI: 10.3389/fnagi.2019.00204
Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects
Abstract
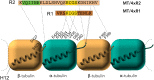
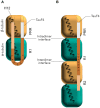
Microtubules (MTs) play a fundamental role in many vital processes such as cell division and neuronal activity. They are key structural and functional elements in axons, supporting neurite differentiation and growth, as well as transporting motor proteins along the axons, which use MTs as support tracks. Tau is a stabilizing MT associated protein, whose functions are mainly regulated by phosphorylation. A disruption of the MT network, which might be caused by Tau loss of function, is observed in a group of related diseases called tauopathies, which includes Alzheimer's disease (AD). Tau is found hyperphosphorylated in AD, which might account for its loss of MT stabilizing capacity. Since destabilization of MTs after dissociation of Tau could contribute to toxicity in neurodegenerative diseases, a molecular understanding of this interaction and its regulation is essential.
Keywords: Alzheimer’s disease; biophysical methods; intrinsically disordered proteins; neurodegenerative diseases; post-translational modifications.
Figures
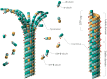


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

