Specific Inhibition of CYP4A Alleviates Myocardial Oxidative Stress and Apoptosis Induced by Advanced Glycation End-Products
- PMID: 31447674
- PMCID: PMC6696796
- DOI: 10.3389/fphar.2019.00876
Specific Inhibition of CYP4A Alleviates Myocardial Oxidative Stress and Apoptosis Induced by Advanced Glycation End-Products
Abstract
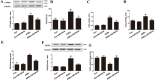
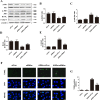
High exposure to advanced glycation end-products (AGEs) may induce cardiotoxicity. However, the effects and mechanisms remain to be further clarified. CYP4A plays an important role in the pathophysiological process of myocardial abnormalities by modulating oxidative stress and apoptosis (OS/Apop) signaling pathway. The present work aimed to investigate whether CYP4A mediates AGEs-induced myocardial injury. AGEs solution was administered intragastrically to C57BL/6 mice for 60 days, while the specific inhibitor of CYP4A, HET0016, was given from the 47th day via intraperitoneal injection for 2 weeks. Levels of OS/Apop in heart tissue were measured. The effects on the cell viability and apoptosis were detected in primary rat cardiomyocytes. To further investigate the mechanism, H9c2 cells were treated with HET0016 or small interfering RNAs (siRNAs) against CYP4a mRNA before incubation with AGEs. Exposure to AGEs led to significantly increased expression of CYP4A and levels of OS/Apop in heart and H9c2 cells both in vivo and in vitro. The OS/Apop pathway was activated with increased expression of NOX2, p-JNK, and cleaved caspase-3 (c-caspase-3) and decreased expression of p-Akt and Bcl-xL both in vivo and in vitro. Specific CYP4A suppression by HET0016 or siRNA exerted significant protective effects by attenuating AGEs-induced OS/Apop pathways in vitro. Our results demonstrate that specific inhibition of CYP4A might be a potential therapeutic option for myocardial injury induced by AGEs.
Keywords: CYP4A; advanced glycation end-products; apoptosis; myocardium; oxidative stress.
Figures




References
-
- Adamopoulos C., Piperi C., Gargalionis A. N., Dalagiorgou G., Spilioti E., Korkolopoulou P., et al. (2016). Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-kappaB and JNK-AP-1 signaling pathways. Cell. Mol. Life Sci. 73 (8), 1685–1698. 10.1007/s00018-015-2091-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

