Dependence on Dectin-1 Varies With Multiple Candida Species
- PMID: 31447813
- PMCID: PMC6691182
- DOI: 10.3389/fmicb.2019.01800
Dependence on Dectin-1 Varies With Multiple Candida Species
Abstract
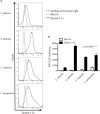
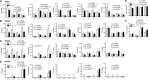
Four Candida spp. (albicans, glabrata, tropicalis, parapsilosis) cause >95% of invasive Candida infections. C. albicans elicits immune responses via pathogen recognition receptors including C-type lectin-like receptors (CLRs). The CLR, Dectin-1 is important for host immunity to C. albicans and C. glabrata, however, whether Dectin-1 is important for host defense against C. tropicalis or C. parapsilosis is unknown. Therefore, we compared the involvement of Dectin-1 in response to these four diverse Candida spp. We found that Dectin-1 mediates innate cytokine responses to these Candida spp. in a species- and cell-dependent manner. Dectin-1 KO mice succumbed to infection with highly virulent C. albicans while they mostly survived infection with less virulent Candida spp. However, Dectin-1 KO mice displayed increased fungal burden following infection with each Candida spp. Additionally, T cells from Dectin-1 KO mice displayed enhanced effector functions likely due to the inability of Dectin-1 KO mice to clear the infections. Together, these data indicate that Dectin-1 is important for host defense to multiple Candida spp., although the specific roles for Dectin-1 varies with different Candida spp.
Keywords: Candida spp.; Dectin-1; T cells; dendritic cells; macrophages.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

