Neural Recording and Modulation Technologies
- PMID: 31448131
- PMCID: PMC6707077
- DOI: 10.1038/natrevmats.2016.93
Neural Recording and Modulation Technologies
Abstract
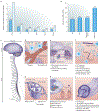
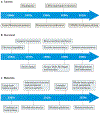
Within the mammalian nervous system, billions of neurons connected by quadrillions of synapses exchange electrical, chemical and mechanical signals. Disruptions to this network manifest as neurological or psychiatric conditions. Despite decades of neuroscience research, our ability to treat or even to understand these conditions is limited by the tools capable of probing the signalling complexity of the nervous system. Although orders of magnitude smaller and computationally faster than neurons, conventional substrate-bound electronics do not address the chemical and mechanical properties of neural tissue. This mismatch results in a foreign-body response and the encapsulation of devices by glial scars, suggesting that the design of an interface between the nervous system and a synthetic sensor requires additional materials innovation. Advances in genetic tools for manipulating neural activity have fuelled the demand for devices capable of simultaneous recording and controlling individual neurons at unprecedented scales. Recently, flexible organic electronics and bio- and nanomaterials have been developed for multifunctional and minimally invasive probes for long-term interaction with the nervous system. In this Review, we discuss the design lessons from the quarter-century-old field of neural engineering, highlight recent materials-driven progress in neural probes, and look at emergent directions inspired by the principles of neural transduction.
Conflict of interest statement
Competing financial interests The authors declare no competing financial interests.
Figures




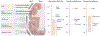

References
-
- Dorsey ER, et al., Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology, 2007. 68(5): p. 384–6. - PubMed
-
- Adelman G, Rane SG, and Villa KF, The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ, 2013. 16(5): p. 639–47. - PubMed
-
- Greenberg PE, et al., The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry, 2003. 64(12): p. 1465–75. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
