The Unusual Properties of Polytetrafluoroethylene Enable Massive-Volume Vitrification of Stem Cells with Low-Concentration Cryoprotectants
- PMID: 31448319
- PMCID: PMC6707752
- DOI: 10.1002/admt.201800289
The Unusual Properties of Polytetrafluoroethylene Enable Massive-Volume Vitrification of Stem Cells with Low-Concentration Cryoprotectants
Abstract
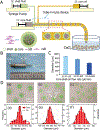
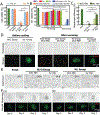
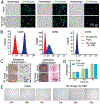
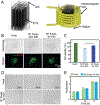
Injectable stem cell-hydrogel constructs hold great potential for regenerative medicine and cell-based therapies. However, their clinical application is still challenging due to their short shelf-life at ambient temperature and the time-consuming fabrication procedure. Banking the constructs at cryogenic temperature may offer the possibility of "off-the-shelf" availability to end-users. However, ice formation during the cryopreservation process may compromise the construct quality and cell viability. Vitrification, cooling biological samples without apparent ice formation, has been explored to resolve the challenge. However, contemporary vitrification methods are limited to very small volume (up to ~0.25 ml) and/or need highly toxic and high concentration (up to ~8 M) of permeable cryoprotectants (pCPAs). Here, we show that polytetrafluoroethylene (PTFE, best known as Teflon for making non-stick cookware) capillary is flexible and unusually stable at a cryogenic temperature. By using the PTFE capillary as a flexible cryopreservation vessel together with alginate hydrogel microencapsulation and Fe3O4 nanoparticle-mediated nanowarming to suppress ice formation, massive-volume (10 ml) vitrification of cell-alginate hydrogel constructs with a low concentration (~2.5 M) of pCPA can be achieved. This may greatly facilitate the use of stem cell-based constructs for tissue regeneration and cell based therapies in the clinic.
Keywords: Fe3O4 nanoparticles; PTFE; alginate hydrogel constructs; low-CPA vitrification.
Figures



Similar articles
-
Dual Suppression Effect of Magnetic Induction Heating and Microencapsulation on Ice Crystallization Enables Low-Cryoprotectant Vitrification of Stem Cell-Alginate Hydrogel Constructs.ACS Appl Mater Interfaces. 2018 May 16;10(19):16822-16835. doi: 10.1021/acsami.8b04496. Epub 2018 May 7. ACS Appl Mater Interfaces. 2018. PMID: 29688697 Free PMC article.
-
Alginate Hydrogel Microencapsulation Inhibits Devitrification and Enables Large-Volume Low-CPA Cell Vitrification.Adv Funct Mater. 2015 Nov 25;25(44):6939-6850. doi: 10.1002/adfm.201503047. Adv Funct Mater. 2015. PMID: 26640426 Free PMC article.
-
Microencapsulation Facilitates Low-Cryoprotectant Vitrification of Human Umbilical Vein Endothelial Cells.ACS Biomater Sci Eng. 2019 Oct 14;5(10):5273-5283. doi: 10.1021/acsbiomaterials.9b00726. Epub 2019 Sep 19. ACS Biomater Sci Eng. 2019. PMID: 33455232
-
Cryopreservation: Vitrification and Controlled Rate Cooling.Methods Mol Biol. 2017;1590:41-77. doi: 10.1007/978-1-4939-6921-0_5. Methods Mol Biol. 2017. PMID: 28353262 Review.
-
Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures.Cryobiology. 2017 Jun;76:74-91. doi: 10.1016/j.cryobiol.2017.04.004. Epub 2017 Apr 18. Cryobiology. 2017. PMID: 28428046 Review.
Cited by
-
High-performance printed electronics based on inorganic semiconducting nano to chip scale structures.Nano Converg. 2020 Oct 9;7(1):33. doi: 10.1186/s40580-020-00243-6. Nano Converg. 2020. PMID: 33034776 Free PMC article. Review.
-
Vitrification and Rewarming of Magnetic Nanoparticle-Loaded Rat Hearts.Adv Mater Technol. 2022 Mar;7(3):2100873. doi: 10.1002/admt.202100873. Epub 2021 Oct 1. Adv Mater Technol. 2022. PMID: 35668819 Free PMC article.
-
Conduction Cooling and Plasmonic Heating Dramatically Increase Droplet Vitrification Volumes for Cell Cryopreservation.Adv Sci (Weinh). 2021 Apr 10;8(11):2004605. doi: 10.1002/advs.202004605. eCollection 2021 Jun. Adv Sci (Weinh). 2021. PMID: 34141523 Free PMC article.
-
Development of a Vitrification Preservation Process for Bioengineered Epithelial Constructs.Cells. 2022 Mar 25;11(7):1115. doi: 10.3390/cells11071115. Cells. 2022. PMID: 35406679 Free PMC article.
-
Dimethyl sulfoxide in cryopreserved mesenchymal stromal cell therapy products: is there a safety risk to patients?J Transl Med. 2025 Aug 18;23(1):932. doi: 10.1186/s12967-025-06807-6. J Transl Med. 2025. PMID: 40826088 Free PMC article. Review.
References
-
- Dufrane D, Goebbels RM, Gianello P, Transplantation 2010, 90, 1054. - PubMed
- Albani D, Gloria A, Giordano C, Rodilossi S, Russo T, D’Amora U, Tunesi M, Cigada A, Ambrosio L, Forloni G, Thescientificworldjournal 2013, 2013, 270260. - PMC - PubMed
- Chen G, Li J, Song M, Wu Z, Zhang W, Wang Z, Gao J, Yang Z, Ou C, Advanced Functional Materials 2017, 27, 1701798
- Nguyen DK, Son YM, Lee NE, Advanced Healthcare Materials 2015, 4, 1537. - PubMed
- Moshaverinia A, Chen C, Xu X, Ansari S, Zadeh HH, Schricker SR, Paine ML, Moradianoldak J, Khademhosseini A, Snead ML, Advanced Functional Materials 2015, 25, 2296. - PMC - PubMed
-
- Stubban C, Katkov I, Loring JF, Wesselschmidt RL, Human Stem Cell Manual 2007, 47.
-
- Iwatani M, Ikegami K, Kremenska Y, Hattori N, Tanaka S, Yagi S, Shiota K, Stem Cells 2006, 24, 2549. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
