Efficient transplacental IgG transfer in women infected with Zika virus during pregnancy
- PMID: 31449521
- PMCID: PMC6730934
- DOI: 10.1371/journal.pntd.0007648
Efficient transplacental IgG transfer in women infected with Zika virus during pregnancy
Abstract
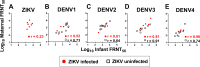
Zika virus (ZIKV) is a newly-identified infectious cause of congenital disease. Transplacental transfer of maternal IgG to the fetus plays an important role in preventing many neonatal infections. However, antibody transfer may also have negative consequences, such as mediating enhancement of flavivirus infections in early life, or trafficking of virus immune complexes to the fetal compartment. ZIKV infection produces placental pathology which could lead to impaired IgG transfer efficiency as occurs in other maternal infections, such as HIV-1 and malaria. In this study, we asked whether ZIKV infection during pregnancy impairs transplacental transfer of IgG. We enrolled pregnant women with fever or rash in a prospective cohort in Vitoria, Brazil during the recent ZIKV epidemic. ZIKV and dengue virus (DENV)-specific IgG, ZIKV and DENV neutralizing antibodies, and routine vaccine antigen-specific IgG were measured in maternal samples collected around delivery and 20 paired cord blood samples. We concluded that 8 of these mothers were infected with ZIKV during pregnancy and 12 were ZIKV-uninfected. The magnitude of flavivirus-specific IgG, neutralizing antibody, and vaccine-elicited IgG were highly correlated between maternal plasma and infant cord blood in both ZIKV-infected and -uninfected mother-infant pairs. Moreover, there was no difference in the magnitude of plasma flavivirus-specific IgG levels between mothers and infants regardless of ZIKV infection status. Our data suggests that maternal ZIKV infection during pregnancy does not impair the efficiency of placental transfer of flavivirus-specific, functional, and vaccine-elicited IgG. These findings have implications for the neonatal outomes of maternal ZIKV infection and optimal administration of antibody-based ZIKV vaccines and therapeutics.
Conflict of interest statement
TS, CAL, CG, MLD, HLI, HJH., PMV, AH, RD, HSW, HKR, and HML all certify no potential conflicts of interest. SRP is serving as a consultant for vaccine programs at Merck, Pfizer, and Moderna. GKS is on the scientific advisory board for investigational vaccine products with Saol Therapeutics and GlaxoSmithKline, the Chair of Data Safety and Monitoring Board with Pfizer, and the site PI at Duke for testing investigational vaccines and products with Novavax, Regeneron, and GlaxoSmithKline/Novartis. In the past three years, RGC has served as a consultant for Arsanis, Basilea, Bayer, Cempra, Contrafect, Meiji Seika Pharma Co., Melinta, Merck, Motif, Paratek, Parion Sciences, Quintiles, Regeneron, SCPharma, The Medicines Company, and Theravance. Moreover, RGC has served on the Adjudication Committee at Bio2 Medical and Novella, the scientific advisory board of Medtronic and Tetraphase, and on the Mortality Board of Pfizer.
Figures




References
-
- Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—U.S. Territories and Freely Associated States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67: 858–867. 10.15585/mmwr.mm6731e1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

