Choice of the initial antiretroviral treatment for HIV-positive individuals in the era of integrase inhibitors
- PMID: 31449566
- PMCID: PMC6709901
- DOI: 10.1371/journal.pone.0221598
Choice of the initial antiretroviral treatment for HIV-positive individuals in the era of integrase inhibitors
Abstract
Background: We aimed to describe the most frequently prescribed initial antiretroviral therapy (ART) regimens in recent years in HIV-positive persons in the Cohort of the Spanish HIV/AIDS Research Network (CoRIS) and to investigate factors associated with the choice of each regimen.
Methods: We analyzed initial ART regimens prescribed in adults participating in CoRIS from 2014 to 2017. Only regimens prescribed in >5% of patients were considered. We used multivariable multinomial regression to estimate Relative Risk Ratios (RRRs) for the association between sociodemographic and clinical characteristics and the choice of the initial regimen.
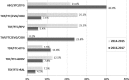
Results: Among 2874 participants, abacavir(ABC)/lamivudine(3TC)/dolutegavir(DTG) was the most frequently prescribed regimen (32.1%), followed by tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC)/elvitegravir(EVG)/cobicistat(COBI) (14.9%), TDF/FTC/rilpivirine (RPV) (14.0%), tenofovir alafenamide (TAF)/FTC/EVG/COBI (13.7%), TDF/FTC+DTG (10.0%), TDF/FTC+darunavir/ritonavir or darunavir/cobicistat (bDRV) (9.8%) and TDF/FTC+raltegravir (RAL) (5.6%). Compared with ABC/3TC/DTG, starting TDF/FTC/RPV was less likely in patients with CD4<200 cells/μL and HIV-RNA>100.000 copies/mL. TDF/FTC+DTG was more frequent in those with CD4<200 cells/μL and HIV-RNA>100.000 copies/mL. TDF/FTC+RAL and TDF/FTC+bDRV were also more frequent among patients with CD4<200 cells//μL and with transmission categories other than men who have sex with men. Compared with ABC/3TC/DTG, the prescription of other initial ART regimens decreased from 2014-2015 to 2016-2017 with the exception of TDF/FTC+DTG. Differences in the choice of the initial ART regimen were observed by hospitals' location.
Conclusions: The choice of initial ART regimens is consistent with Spanish guidelines' recommendations, but is also clearly influenced by physician's perception based on patient's clinical and sociodemographic variables and by the prescribing hospital location.
Conflict of interest statement
IJ has received teaching fees from ViiV Healthcare and has been an external evaluator of scientific projects for GILEAD. ISG has received conference grants or speaker fees from Bristol-Myers Squibb, ViiV Healthcare, Merck Sharp & Dohme and Gilead. SM has been involved in speaking activities and has received grants for research from Abbott, Boehringer & Ingelheim, Bristol-Myers Squibb, Gilead, Glaxo Smith Kline, Janssen Cilag, Merck Sharp & Dohme, Pfizer, Roche, and Schering Plough. All other authors declare no conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Panel de expertos de Gesida y Plan Nacional sobre el SIDA (2017) Documento de consenso de GeSIDA/Plan Nacional sobre el Sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (Actualización enero 2017).
-
- European AIDS Clinical Society (2017) EACS Guidelines. Version 9.0. October 2017.
-
- Pannel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV (update October 25, 2018). Department of Health and Human Services.
-
- GeSIDA/Plan Nacional sobre el SIDA Documento de consenso de GeSIDA/Plan Nacional sobre el SIDA respecto al tratamiento antirretroviral en adultos infectados por del virus de la inmunodeficiencia humana (actualización Enero 2018).
-
- Viciana P, Ocampo A, Hevia H, Palazuelos M, Ledesma F (2014) [Initial antiretroviral treatment in human immunodeficiency virus-infected patients in Spain: Decisions made in relation to particular immunovirological characteristics (PERFIL-es study)]. Enferm Infecc Microbiol Clin 32: 93–95. 10.1016/j.eimc.2013.08.002 - DOI - PubMed