Molecular-Level Interactions between Engineered Materials and Cells
- PMID: 31450647
- PMCID: PMC6747072
- DOI: 10.3390/ijms20174142
Molecular-Level Interactions between Engineered Materials and Cells
Abstract
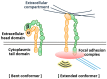
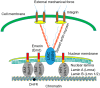
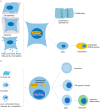
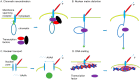
Various recent experimental observations indicate that growing cells on engineered materials can alter their physiology, function, and fate. This finding suggests that better molecular-level understanding of the interactions between cells and materials may guide the design and construction of sophisticated artificial substrates, potentially enabling control of cells for use in various biomedical applications. In this review, we introduce recent research results that shed light on molecular events and mechanisms involved in the interactions between cells and materials. We discuss the development of materials with distinct physical, chemical, and biological features, cellular sensing of the engineered materials, transfer of the sensing information to the cell nucleus, subsequent changes in physical and chemical states of genomic DNA, and finally the resulting cellular behavior changes. Ongoing efforts to advance materials engineering and the cell-material interface will eventually expand the cell-based applications in therapies and tissue regenerations.
Keywords: cell surface sensors; cellular responses; genome states; materials engineering; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Liu Y., Conaway L., Rutherford Bethard J., Al-Ayoubi A.M., Thompson Bradley A., Zheng H., Weed S.A., Eblen S.T. Phosphorylation of the alternative mRNA splicing factor 45 (SPF45) by Clk1 regulates its splice site utilization, cell migration and invasion. Nucleic Acids Res. 2013;41:4949–4962. doi: 10.1093/nar/gkt170. - DOI - PMC - PubMed
-
- Yao C.H., Liu G.Y., Wang R., Moon S.H., Gross R.W., Patti G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of beta-oxidation. PLoS Biol. 2018;16:e2003782. doi: 10.1371/journal.pbio.2003782. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

