Immunogenicity and Protection Efficacy of a Naked Self-Replicating mRNA-Based Zika Virus Vaccine
- PMID: 31450775
- PMCID: PMC6789535
- DOI: 10.3390/vaccines7030096
Immunogenicity and Protection Efficacy of a Naked Self-Replicating mRNA-Based Zika Virus Vaccine
Abstract
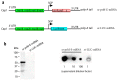
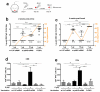
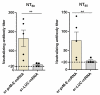
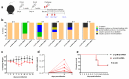
To combat emerging infectious diseases like Zika virus (ZIKV), synthetic messenger RNAs (mRNAs) encoding viral antigens are very attractive as they allow a rapid, generic, and flexible production of vaccines. In this work, we engineered a self-replicating mRNA (sr-mRNA) vaccine encoding the pre-membrane and envelope (prM-E) glycoproteins of ZIKV. Intradermal electroporation of as few as 1 µg of this mRNA-based ZIKV vaccine induced potent humoral and cellular immune responses in BALB/c and especially IFNAR1-/- C57BL/6 mice, resulting in a complete protection of the latter mice against ZIKV infection. In wild-type C57BL/6 mice, the vaccine resulted in very low seroconversion rates and antibody titers. The potency of the vaccine was inversely related to the dose of mRNA used in wild-type BALB/c or C57BL/6 mice, as robust type I interferon (IFN) response was determined in a reporter mice model (IFN-β+/Δβ-luc). We further investigated the inability of the sr-prM-E-mRNA ZIKV vaccine to raise antibodies in wild-type C57BL/6 mice and found indications that type I IFNs elicited by this naked sr-mRNA vaccine might directly impede the induction of a robust humoral response. Therefore, we assume that the efficacy of sr-mRNA vaccines after intradermal electroporation might be increased by strategies that temper their inherent innate immunogenicity.
Keywords: IFNAR1 knockout mice; Zika virus vaccine; self-replicating mRNA; type I interferon response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Oehler E., Watrin L., Larre P., Leparc-Goffart I., Lastere S., Valour F., Baudouin L., Mallet H., Musso D., Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Eurosurveillance. 2014;19:20720. doi: 10.2807/1560-7917.ES2014.19.9.20720. - DOI - PubMed
-
- Oehler E., Fournier E., Leparc-Goffart I., Larre P., Cubizolle S., Sookhareea C., Lastere S., Ghawche F. Increase in cases of Guillain-Barre syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Eurosurveillance. 2015;20:30079. doi: 10.2807/1560-7917.ES.2015.20.48.30079. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

