HIV-Nef Protein Transfer to Endothelial Cells Requires Rac1 Activation and Leads to Endothelial Dysfunction Implications for Statin Treatment in HIV Patients
- PMID: 31451038
- PMCID: PMC7009312
- DOI: 10.1161/CIRCRESAHA.119.315082
HIV-Nef Protein Transfer to Endothelial Cells Requires Rac1 Activation and Leads to Endothelial Dysfunction Implications for Statin Treatment in HIV Patients
Abstract
Rationale: Even in antiretroviral therapy-treated patients, HIV continues to play a pathogenic role in cardiovascular diseases. A possible cofactor may be persistence of the early HIV response gene Nef, which we have demonstrated recently to persist in the lungs of HIV+ patients on antiretroviral therapy. Previously, we have reported that HIV strains with Nef, but not Nef-deleted HIV strains, cause endothelial proinflammatory activation and apoptosis.
Objective: To characterize mechanisms through which HIV-Nef leads to the development of cardiovascular diseases using ex vivo tissue culture approaches as well as interventional experiments in transgenic murine models.
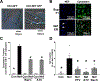
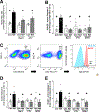
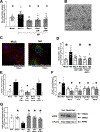
Methods and results: Extracellular vesicles derived from both peripheral blood mononuclear cells and plasma from HIV+ patient blood samples induced human coronary artery endothelial cells dysfunction. Plasma-derived extracellular vesicles from antiretroviral therapy+ patients who were HIV-Nef+ induced significantly greater endothelial apoptosis compared with HIV-Nef-plasma extracellular vesicles. Both HIV-Nef expressing T cells and HIV-Nef-induced extracellular vesicles increased transfer of cytosol and Nef protein to endothelial monolayers in a Rac1-dependent manner, consequently leading to endothelial adhesion protein upregulation and apoptosis. HIV-Nef induced Rac1 activation also led to dsDNA breaks in endothelial colony forming cells, thereby resulting in endothelial colony forming cell premature senescence and endothelial nitric oxide synthase downregulation. These Rac1-dependent activities were characterized by NOX2-mediated reactive oxygen species production. Statin treatment equally inhibited Rac1 inhibition in preventing or reversing all HIV-Nef-induction abnormalities assessed. This was likely because of the ability of statins to block Rac1 prenylation as geranylgeranyl transferase inhibitors were effective in inhibiting HIV-Nef-induced reactive oxygen species formation. Finally, transgenic expression of HIV-Nef in endothelial cells in a murine model impaired endothelium-mediated aortic ring dilation, which was then reversed by 3-week treatment with 5 mg/kg atorvastatin.
Conclusions: These studies establish a mechanism by which HIV-Nef persistence despite antiretroviral therapy could contribute to ongoing HIV-related vascular dysfunction, which may then be ameliorated by statin treatment.
Keywords: apoptosis; cardiovascular diseases; endothelial progenitor cells; extracellular vesicles; statin.
Figures





Comment in
-
Of Vesicles and Viruses: Why Statins Are Good for HIV Patients.Circ Res. 2019 Oct 11;125(9):821-823. doi: 10.1161/CIRCRESAHA.119.315908. Epub 2019 Oct 10. Circ Res. 2019. PMID: 31600131 No abstract available.
References
-
- Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, Rimland D, Bedimo R, Goetz MB, Rodriguez-Barradas MC, Crane HM, Gibert CL, Brown ST, Tindle HA, Warner AL, Alcorn C, Skanderson M, Justice AC, Freiberg MS. Hiv infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209–216 - PMC - PubMed
-
- Francisci D, Giannini S, Baldelli F, Leone M, Belfiori B, Guglielmini G, Malincarne L, Gresele P. Hiv type 1 infection, and not short-term haart, induces endothelial dysfunction. AIDS. 2009;23:589–596 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

