Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function
- PMID: 31451516
- PMCID: PMC7011869
- DOI: 10.1212/WNL.0000000000008168
Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function
Abstract
Objective: To study vamorolone, a first-in-class steroidal anti-inflammatory drug, in Duchenne muscular dystrophy (DMD).
Methods: An open-label, multiple-ascending-dose study of vamorolone was conducted in 48 boys with DMD (age 4-<7 years, steroid-naive). Dose levels were 0.25, 0.75, 2.0, and 6.0 mg/kg/d in an oral suspension formulation (12 boys per dose level; one-third to 10 times the glucocorticoid dose in DMD). The primary goal was to define optimal doses of vamorolone. The primary outcome for clinical efficacy was time to stand from supine velocity.
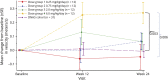
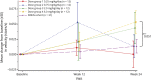
Results: Oral administration of vamorolone at all doses tested was safe and well tolerated over the 24-week treatment period. The 2.0-mg/kg/d dose group met the primary efficacy outcome of improved muscle function (time to stand; 24 weeks of vamorolone treatment vs natural history controls), without evidence of most adverse effects of glucocorticoids. A biomarker of bone formation, osteocalcin, increased in vamorolone-treated boys, suggesting possible loss of bone morbidities seen with glucocorticoids. Biomarker outcomes for adrenal suppression and insulin resistance were also lower in vamorolone-treated patients with DMD relative to published studies of glucocorticoid therapy.
Conclusions: Daily vamorolone treatment suggested efficacy at doses of 2.0 and 6.0 mg/kg/d in an exploratory 24-week open-label study.
Classification of evidence: This study provides Class IV evidence that for boys with DMD, vamorolone demonstrated possible efficacy compared to a natural history cohort of glucocorticoid-naive patients and appeared to be tolerated.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- The Nobel Prize in Physiology or Medicine 1950. Nobelprize.org. Nobel Media AB. 2014. Available at: nobelprize.org/nobel_prizes/medicine/laureates/1950/. Accessed May 27, 2018.
-
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. - PubMed
-
- McDonald CM, Henricson EK, Abresch RT, et al. . Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 2018;391:451–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
