The Host Immune System Facilitates Disseminated Staphylococcus aureus Disease Due to Phagocytic Attraction to Candida albicans during Coinfection: a Case of Bait and Switch
- PMID: 31451623
- PMCID: PMC6803350
- DOI: 10.1128/IAI.00137-19
The Host Immune System Facilitates Disseminated Staphylococcus aureus Disease Due to Phagocytic Attraction to Candida albicans during Coinfection: a Case of Bait and Switch
Abstract
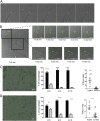
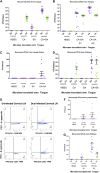
Invasive Staphylococcus aureus infections account for 15 to 50% of fatal bloodstream infections annually. These disseminated infections often arise without a defined portal of entry into the host but cause high rates of mortality. The fungus Candida albicans and the Gram-positive bacterium S. aureus can form polymicrobial biofilms on epithelial tissue, facilitated by the C. albicans adhesin encoded by ALS3 While a bacterium-fungus interaction is required for systemic infection, the mechanism by which bacteria disseminate from the epithelium to internal organs is unclear. In this study, we show that highly immunogenic C. albicans hyphae attract phagocytic cells, which rapidly engulf adherent S. aureus and subsequently migrate to cervical lymph nodes. Following S. aureus-loaded phagocyte translocation from the mucosal surface, S. aureus produces systemic disease with accompanying morbidity and mortality. Our results suggest a novel role for the host in facilitating a bacterium-fungus infectious synergy, leading to disseminated staphylococcal disease.
Keywords: Candida albicans; Staphylococcus aureus; biofilms; innate immunity; polymicrobial.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
Candida albicans Augments Staphylococcus aureus Virulence by Engaging the Staphylococcal agr Quorum Sensing System.mBio. 2019 Jun 4;10(3):e00910-19. doi: 10.1128/mBio.00910-19. mBio. 2019. PMID: 31164467 Free PMC article.
-
Staphylococcus-Candida Interaction Models: Antibiotic Resistance Testing and Host Interactions.Methods Mol Biol. 2016;1356:153-61. doi: 10.1007/978-1-4939-3052-4_11. Methods Mol Biol. 2016. PMID: 26519071
-
Morphology-Independent Virulence of Candida Species during Polymicrobial Intra-abdominal Infections with Staphylococcus aureus.Infect Immun. 2015 Oct 19;84(1):90-8. doi: 10.1128/IAI.01059-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26483410 Free PMC article.
-
Metabolic Adaptations During Staphylococcus aureus and Candida albicans Co-Infection.Front Immunol. 2021 Dec 8;12:797550. doi: 10.3389/fimmu.2021.797550. eCollection 2021. Front Immunol. 2021. PMID: 34956233 Free PMC article. Review.
-
The great escape: pathogen versus host.PLoS Pathog. 2015 Mar 12;11(3):e1004661. doi: 10.1371/journal.ppat.1004661. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25764299 Free PMC article. Review. No abstract available.
Cited by
-
Candida-bacterial cross-kingdom interactions.Trends Microbiol. 2023 Dec;31(12):1287-1299. doi: 10.1016/j.tim.2023.08.003. Epub 2023 Aug 26. Trends Microbiol. 2023. PMID: 37640601 Free PMC article. Review.
-
How Biofilm Growth Affects Candida-Host Interactions.Front Microbiol. 2020 Jun 25;11:1437. doi: 10.3389/fmicb.2020.01437. eCollection 2020. Front Microbiol. 2020. PMID: 32670252 Free PMC article. Review.
-
Interactions between Candida albicans and the resident microbiota.Front Microbiol. 2022 Sep 20;13:930495. doi: 10.3389/fmicb.2022.930495. eCollection 2022. Front Microbiol. 2022. PMID: 36204612 Free PMC article. Review.
-
Recent Advances and Opportunities in the Study of Candida albicans Polymicrobial Biofilms.Front Cell Infect Microbiol. 2022 Feb 18;12:836379. doi: 10.3389/fcimb.2022.836379. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35252039 Free PMC article. Review.
-
Host-mycobiome metabolic interactions in health and disease.Gut Microbes. 2022 Jan-Dec;14(1):2121576. doi: 10.1080/19490976.2022.2121576. Gut Microbes. 2022. PMID: 36151873 Free PMC article.
References
-
- Deveau A, Bonito G, Uehling J, Paoletti M, Becker M, Bindschedler S, Hacquard S, Herve V, Labbe J, Lastovetsky OA, Mieszkin S, Millet LJ, Vajna B, Junier P, Bonfante P, Krom BP, Olsson S, van Elsas JD, Wick LY. 2018. Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev 42:335–352. doi:10.1093/femsre/fuy008. - DOI - PubMed
-
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi:10.1093/cid/ciw691. - DOI - PMC - PubMed
-
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi:10.1056/NEJMoa1306801. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

