Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study
- PMID: 31451630
- PMCID: PMC6744844
- DOI: 10.1073/pnas.1906929116
Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study
Abstract
Biocompatible gold nanoparticles designed to absorb light at wavelengths of high tissue transparency have been of particular interest for biomedical applications. The ability of such nanoparticles to convert absorbed near-infrared light to heat and induce highly localized hyperthermia has been shown to be highly effective for photothermal cancer therapy, resulting in cell death and tumor remission in a multitude of preclinical animal models. Here we report the initial results of a clinical trial in which laser-excited gold-silica nanoshells (GSNs) were used in combination with magnetic resonance-ultrasound fusion imaging to focally ablate low-intermediate-grade tumors within the prostate. The overall goal is to provide highly localized regional control of prostate cancer that also results in greatly reduced patient morbidity and improved functional outcomes. This pilot device study reports feasibility and safety data from 16 cases of patients diagnosed with low- or intermediate-risk localized prostate cancer. After GSN infusion and high-precision laser ablation, patients underwent multiparametric MRI of the prostate at 48 to 72 h, followed by postprocedure mpMRI/ultrasound targeted fusion biopsies at 3 and 12 mo, as well as a standard 12-core systematic biopsy at 12 mo. GSN-mediated focal laser ablation was successfully achieved in 94% (15/16) of patients, with no significant difference in International Prostate Symptom Score or Sexual Health Inventory for Men observed after treatment. This treatment protocol appears to be feasible and safe in men with low- or intermediate-risk localized prostate cancer without serious complications or deleterious changes in genitourinary function.
Keywords: MRI-ultrasound fusion; focal therapy; gold nanoshell; photothermal therapy; prostate cancer.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: A.R.R. is the national principal investigator for the multiinstitutional trial of GSN-directed ablation, funded by Nanospectra Biosciences. He is also a consultant for Nanospectra Biosciences. J.L.W. and N.J.H. cofounded Nanospectra Biosciences in 2001 to transfer the photothermal therapeutics process from their labs into the clinic. They both have a small equity stake in this company but are not involved in any way with the company’s business or strategic decisions.
Figures
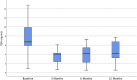

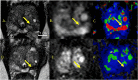
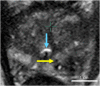
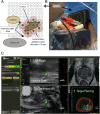
Comment in
-
Re: Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study.J Urol. 2020 Jan;203(1):31. doi: 10.1097/JU.0000000000000614. Epub 2019 Oct 21. J Urol. 2020. PMID: 31634071 No abstract available.
References
-
- Mie G., Articles on the optical characteristics of turbid tubes, especially colloidal metal solutions. Ann. Phys. Berlin 25, 377–445 (1908).
-
- Aden A. L., Kerker M., Scattering of electromagnetic waves from 2 concentric spheres. J. Appl. Phys. 22, 1242–1246 (1951).
-
- Neeves A. E., Birnboim M. H., Composite structures for the enhancement of nonlinear-optical susceptibility. J. Opt. Soc. Am. B 6, 787–796 (1989). - PubMed
-
- Averitt R. D., Sarkar D., Halas N. J., Plasmon resonance shifts of Au-coated Au2S nanoshells: Insight into multicomponent nanoparticle growth. Phys. Rev. Lett. 78, 4217–4220 (1997).
-
- Zhou H. S., Honma I., Komiyama H., Haus J. W., Controlled synthesis and quantum-size effect in gold-coated nanoparticles. Phys. Rev. B Condens. Matter 50, 12052–12056 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

