Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents
- PMID: 31451718
- PMCID: PMC6710276
- DOI: 10.1038/s41598-019-48584-5
Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents
Abstract
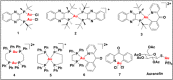
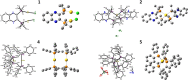
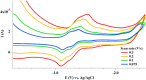
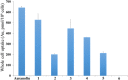
The reaction of gold reagents [HAuCl4•3H2O], [AuCl(tht)], or cyclometalated gold(III) precursor, [C^NAuCl2] with chiral ((R,R)-(-)-2,3-bis(t-butylmethylphosphino) quinoxaline) and non-chiral phosphine (1,2-Bis(diphenylphosphino)ethane, dppe) ligands lead to distorted Au(I), (1, 2, 4, 5) and novel cyclometalated Au(III) complexes (3, 6). These gold compounds were characterized by multinuclear NMR, microanalysis, mass spectrometry, and X-ray crystallography. The inherent electrochemical properties of the gold complexes were also studied by cyclic voltammetry and theoretical insight of the complexes was gained by density functional theory and TD-DFT calculations. The complexes effectively kill cancer cells with IC50 in the range of ~0.10-2.53 μΜ across K562, H460, and OVCAR8 cell lines. In addition, the retinal pigment epithelial cell line, RPE-Neo was used as a healthy cell line for comparison. Differential cellular uptake in cancer cells was observed for the compounds by measuring the intracellular accumulation of gold using ICP-OES. Furthermore, the compounds trigger early - late stage apoptosis through potential disruption of redox homeostasis. Complexes 1 and 3 induce predominant G1 cell cycle arrest. Results presented in this report suggest that stable gold-phosphine complexes with variable oxidation states hold promise in anticancer drug discovery and need further development.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Rackham O, Nichols SJ, Leedman PJ, Berners-Price SJ, Filipovska A. A gold(I) phosphine complex selectively induces apoptosis in breast cancer cells: Implications for anticancer therapeutics targeted to mitochondria. Biochem. Pharmacol. 2007;74:992–1002. doi: 10.1016/j.bcp.2007.07.022. - DOI - PubMed
-
- Berners-Price SJ, et al. In Vivo Antitumor Activity and in Vitro Cytotoxic Properties of Bis[1,2-bis(diphenylphosphino)ethane]gold(I) Chloride. Cancer Res. 1986;46:5486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

