Intrauterine programming of obesity and type 2 diabetes
- PMID: 31451874
- PMCID: PMC6731191
- DOI: 10.1007/s00125-019-4951-9
Intrauterine programming of obesity and type 2 diabetes
Abstract
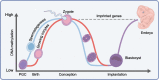
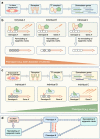
The type 2 diabetes epidemic and one of its predisposing factors, obesity, are major influences on global health and economic burden. It is accepted that genetics and the current environment contribute to this epidemic; however, in the last two decades, both human and animal studies have consolidated considerable evidence supporting the 'developmental programming' of these conditions, specifically by the intrauterine environment. Here, we review the various in utero exposures that are linked to offspring obesity and diabetes in later life, including epidemiological insights gained from natural historical events, such as the Dutch Hunger Winter, the Chinese famine and the more recent Quebec Ice Storm. We also describe the effects of gestational exposure to endocrine disruptors, maternal infection and smoking to the fetus in relation to metabolic programming. Causal evidence from animal studies, motivated by human observations, is also discussed, as well as some of the proposed underlying molecular mechanisms for developmental programming of obesity and type 2 diabetes, including epigenetics (e.g. DNA methylation and histone modifications) and microRNA interactions. Finally, we examine the effects of non-pharmacological interventions, such as improving maternal dietary habits and/or increasing physical activity, on the offspring epigenome and metabolic outcomes.
Keywords: Developmental programming; Epigenetic variation; Intrauterine programming; Life course development; Maternal exposures; MicroRNAs; Obesity; Paternal exposures; Review; Type 2 diabetes.
Figures
References
-
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. - PubMed
-
- Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann N Y Acad Sci. 2010;1212(1):78–96. - PubMed
-
- Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351(9097):173–177. - PubMed
-
- Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(10):787–794. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

