Engineering Geobacillus thermoglucosidasius for direct utilisation of holocellulose from wheat straw
- PMID: 31452680
- PMCID: PMC6701081
- DOI: 10.1186/s13068-019-1540-6
Engineering Geobacillus thermoglucosidasius for direct utilisation of holocellulose from wheat straw
Abstract
Background: A consolidated bioprocessing (CBP), where lignocellulose is converted into the desired product(s) in a single fermentative step without the addition of expensive degradative enzymes, represents the ideal solution of renewable routes to chemicals and fuels. Members of the genus Geobacillus are able to grow at elevated temperatures and are able to utilise a wide range of oligosaccharides derived from lignocellulose. This makes them ideally suited to the development of CBP.
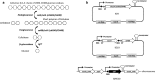
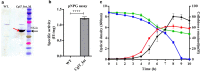
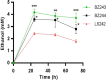
Results: In this study, we engineered Geobacillus thermoglucosidasius NCIMB 11955 to utilise lignocellulosic biomass, in the form of nitric acid/ammonia treated wheat straw to which expensive hydrolytic enzymes had not been added. Two different strains, BZ9 and BZ10, were generated by integrating the cglT (β-1,4-glucosidase) gene from Thermoanaerobacter brockii into the genome, and localising genes encoding different cellulolytic enzymes on autonomous plasmids. The plasmid of strain BZ10 carried a synthetic cellulosomal operon comprising the celA (Endoglucanase A) gene from Clostridium thermocellum and cel6B (Exoglucanase) from Thermobifida fusca; whereas, strain BZ9 contained a plasmid encoding the celA (multidomain cellulase) gene from Caldicellulosiruptor bescii. All of the genes were successfully expressed, and their encoded products secreted in a functionally active form, as evidenced by their detection in culture supernatants by Western blotting and enzymatic assay. In the case of the C. bescii CelA enzyme, this is one of the first times that the heterologous production of this multi-functional enzyme has been achieved in a heterologous host. Both strains (BZ9 and BZ10) exhibited improved growth on pre-treated wheat straw, achieving a higher final OD600 and producing greater numbers of viable cells. To demonstrate that cellulosic ethanol can be produced directly from lignocellulosic biomass by a single organism, we established our consortium of hydrolytic enzymes in a previously engineered ethanologenic G. thermoglucosidasius strain, LS242. We observed approximately twofold and 1.6-fold increase in ethanol production in the recombinant G. thermoglucosidasius equivalent to BZ9 and BZ10, respectively, compared to G. thermoglucosidasius LS242 strain at 24 h of growth.
Conclusion: We engineered G. thermoglucosidasius to utilise a real-world lignocellulosic biomass substrate and demonstrated that cellulosic ethanol can be produced directly from lignocellulosic biomass in one step. Direct conversion of biomass into desired products represents a new paradigm for CBP, offering the potential for carbon neutral, cost-effective production of sustainable chemicals and fuels.
Keywords: Biomass; Cellulases; Consolidated bioprocessing (CBP); Endo/exoglucanases; Geobacillus thermoglucosidasius; Glycoside hydrolases; β-Glucosidase.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315(5813):804–807. - PubMed
-
- Mazzoli R, Lamberti C, Pessione E. Engineering new metabolic capabilities in bacteria: lessons from recombinant cellulolytic strategies. Trends Biotechnol. 2012;30(2):111–119. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

