Construction of industrial Saccharomyces cerevisiae strains for the efficient consolidated bioprocessing of raw starch
- PMID: 31452682
- PMCID: PMC6701143
- DOI: 10.1186/s13068-019-1541-5
Construction of industrial Saccharomyces cerevisiae strains for the efficient consolidated bioprocessing of raw starch
Abstract
Background: Consolidated bioprocessing (CBP) combines enzyme production, saccharification and fermentation into a one-step process. This strategy represents a promising alternative for economic ethanol production from starchy biomass with the use of amylolytic industrial yeast strains.
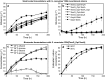
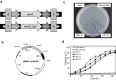
Results: Recombinant Saccharomyces cerevisiae Y294 laboratory strains simultaneously expressing an α-amylase and glucoamylase gene were screened to identify the best enzyme combination for raw starch hydrolysis. The codon optimised Talaromyces emersonii glucoamylase encoding gene (temG_Opt) and the native T. emersonii α-amylase encoding gene (temA) were selected for expression in two industrial S. cerevisiae yeast strains, namely Ethanol Red™ (hereafter referred to as the ER) and M2n. Two δ-integration gene cassettes were constructed to allow for the simultaneous multiple integrations of the temG_Opt and temA genes into the yeasts' genomes. During the fermentation of 200 g l-1 raw corn starch, the amylolytic industrial strains were able to ferment raw corn starch to ethanol in a single step with high ethanol yields. After 192 h at 30 °C, the S. cerevisiae ER T12 and M2n T1 strains (containing integrated temA and temG_Opt gene cassettes) produced 89.35 and 98.13 g l-1 ethanol, respectively, corresponding to estimated carbon conversions of 87 and 94%, respectively. The addition of a commercial granular starch enzyme cocktail in combination with the amylolytic yeast allowed for a 90% reduction in exogenous enzyme dosage, compared to the conventional simultaneous saccharification and fermentation (SSF) control experiment with the parental industrial host strains.
Conclusions: A novel amylolytic enzyme combination has been produced by two industrial S. cerevisiae strains. These recombinant strains represent potential drop-in CBP yeast substitutes for the existing conventional and raw starch fermentation processes.
Keywords: Amylases; Biofuels; Consolidated bioprocessing; Industrial yeast; Raw corn starch; Talaromyces emersonii.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

References
-
- Mobini-Dehkordi M, Javan FA. Application of alpha-amylase biotechnology. J Biol Today’s World. 2012;1:15–20.
-
- Nigam P, Singh D. Enzyme and microbial systems involved in starch processing. Enzyme Microb Technol. 1995;17:770–778. doi: 10.1016/0141-0229(94)00003-A. - DOI
-
- Szymanowska-Powałowska D, Lewandowicz G, Kubiak P, Błaszczak W. Stability of the process of simultaneous saccharification and fermentation of corn flour. The effect of structural changes of starch by stillage recycling and scaling up of the process. Fuel. 2014;119:328–334. doi: 10.1016/j.fuel.2013.11.034. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

