B cell depletion can be effective in multiple sclerosis but failed in a patient with advanced childhood cerebral X-linked adrenoleukodystrophy
- PMID: 31452685
- PMCID: PMC6696829
- DOI: 10.1177/1756286419868133
B cell depletion can be effective in multiple sclerosis but failed in a patient with advanced childhood cerebral X-linked adrenoleukodystrophy
Abstract
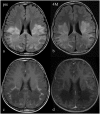
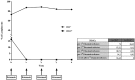
Rituximab exerts its clinical efficacy by its specific pattern of depletion of CD20+ B lymphocytes and it has been demonstrated that rituximab is an effective treatment for relapsing remitting multiple sclerosis. X-linked adrenoleukodystrophy (X-ALD), the most common monogenetic neuroinflammatory disorder, shares substantial overlap with multiple sclerosis in the neuropathological changes found in brain tissues in advanced stages of the disease. While there is no effective therapy for these patients, we hypothesized that rituximab might be effective in arresting the neuroinflammatory process. Our detailed clinical, imaging and immunological data revealed that rituximab is not effective in advanced stages of X-ALD and consequently should not be applied for compassionate use in these patients.
Keywords: B cells; X-ALD; X-linked adrenoleukodystrophy; multiple sclerosis; rituximab.
Conflict of interest statement
Conflict of interest statement: H. Rosewich and S. Nessler report no conflict of interest. W. Brück has received honoraria for lectures by Bayer Vital, Biogen, Merck Serono, Teva, Genzyme, Roche and Novartis. He is a member of scientific advisory boards for Teva, Biogen, Novartis and Genzyme, and receives research support from Teva, Biogen, Genzyme and Novartis. J. Gärtner has received honoraria for lectures and consultancy fees from Bayer, Teva and Novartis and research support from Novartis.
Figures
References
-
- Steinberg SJ, Moser AB, Raymond GV. X-Linked Adrenoleukodystrophy. In: Pagon RA, Adam MP, Ardinger HHet al. (eds) GeneReviews(R). Seattle: University of Washington, 1993. - PubMed
-
- Miller WP, Rothman SM, Nascene Det al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood 2011; 118: 1971–1978. - PubMed
-
- Cartier N, Hacein-Bey-Abina S, Bartholomae CCet al. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol 2012; 507: 187–198. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

