Tumor-induced DNA methylation in the white blood cells of patients with colorectal cancer
- PMID: 31452782
- PMCID: PMC6676401
- DOI: 10.3892/ol.2019.10638
Tumor-induced DNA methylation in the white blood cells of patients with colorectal cancer
Abstract
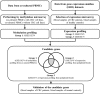
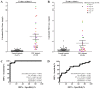
The secretions of cancer cells alter epigenetic regulation in cancer stromal cells. The present study investigated the methylation changes in white blood cells (WBCs) caused by the secretions of colorectal cancer (CRC) cells. Changes in the DNA methylation of peripheral blood mononuclear cells (PBMCs) from normal individuals co-cultured with CRC cells were estimated using a methylation microarray. These changes were then compared against the DNA methylation changes and mRNA levels observed in the WBCs of patients with CRC. Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 (PLOD1) and matrix metalloproteinase 9 (MMP9) were selected to assess the DNA methylation of the WBCs from CRC patients using real-time methylation-specific PCR. The majority of the genes analyzed presented high levels of mRNA in the WBCs of the patients with CRC and DNA methylation in the co-cultured PBMCs. Intragenic methylation revealed the strongest association (P=8.52×10-21). For validation, MMP9 and PLOD1 were selected and used to test WBCs from 32 patients with CRC and 57 normal controls. The intragenic MMP9 methylation was commonly found (P<0.0001) with high sensitivity (90.63%) and high specificity (96.49%), and a positive predictive value of 93.33% and a negative predictive value of 93.22%. PLOD1 methylation was revealed to have lower sensitivity (30.00%) but higher specificity (97.92%). In addition to circulating WBCs, MMP9 protein expression was observed in infiltrating WBCs and the metastatic lymph nodes of patients with CRC. In conclusion, CRC cells secrete factors that induce genome wide DNA methylation changes in the WBCs of patients with CRC. These changes, including intragenic MMP9 methylation in WBCs, are promising CRC biomarkers to be tested in future CRC screening studies.
Keywords: CRC; WBCs; matrix metalloproteinase 9 methylation.
Figures

References
-
- Cooper GM, Hausman RE. The development and causes of cancer. The cell: A molecular approach. (2nd) 2000:725–766.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
