Evaluation of the adverse events following immunization surveillance system in Guruve district, Mashonaland Central 2017
- PMID: 31452827
- PMCID: PMC6693787
- DOI: 10.11604/pamj.2018.31.202.16573
Evaluation of the adverse events following immunization surveillance system in Guruve district, Mashonaland Central 2017
Abstract
Introduction: An adverse event following immunisation is any untoward medical occurrence which follows vaccination. Frequency of adverse events ranges from 13% to 34% and they should be reported regardless of severity. From the beginning of 2016 to mid-2017, Guruve district in Zimbabwe did not report any AEFIs. This suggests the surveillance system may be failing to detect adverse events. We therefore evaluated the AEFI surveillance system in Guruve district.
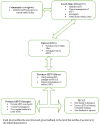
Methods: We conducted a surveillance system evaluation using the updated Centers for Disease Control guidelines for evaluating public health surveillance systems. We interviewed health workers and caregivers of babies under 2 years in Guruve district. We also reviewed all records on AEFI surveillance for the period of January 2016 to November 2017.
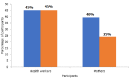
Results: We recruited 31 health workers and 33 caregivers into the study. Between January 2016 and mid-2017, 39% of the caregivers had children who had suffered AEFIs and 45% of the health workers had encountered AEFIs but none had been notified. The main reasons for failure to report AEFIs included health workers' fear of personal consequences and caregivers thinking that an adverse event was not serious enough to report. Knowledge of the surveillance system was good amongst the majority of health workers. All the resources needed by the surveillance system were available.
Conclusion: We concluded that health workers in Guruve district were afraid to report adverse events following immunization and caregivers were reluctant to report mild adverse events hence the surveillance system was performing poorly and was not useful. However, the stability of the system and the good knowledge gives a good foundation for improving the surveillance system.
Keywords: AEFI; Guruve; Zimbabwe; surveillance; vaccination.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO . Global Vaccine Safety. Adverse events following immunization (AEFI); Accessed 16 May 2018.
-
- General Practice Notebook . Adverse events following immunisation (AEFIs) Accessed 16 May 2018.
-
- Edwards KM, Meade BD, Decker MD, Reed GF, Rennels MB, Steinhoff MC, et al. Comparison of 13 Acellular Pertussis Vaccines: overview and serologic response. Pediatrics. 1995;96(3 Pt 2):548–557. - PubMed
-
- WHO . Immunization, Vaccines and Biologicals. SAGE issues its 2018 assessment report of the Global Vaccine Action Plan. Global Vaccine Action Plan; Accessed 16 May 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical