Binding and Catalytic Mechanisms of Veratryl Alcohol Oxidation by Lignin Peroxidase: A Theoretical and Experimental Study
- PMID: 31452859
- PMCID: PMC6700493
- DOI: 10.1016/j.csbj.2019.07.002
Binding and Catalytic Mechanisms of Veratryl Alcohol Oxidation by Lignin Peroxidase: A Theoretical and Experimental Study
Abstract
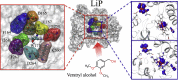
Lignin peroxidase (LiP) and its natural substrate veratryl alcohol (VA) play a crucial role in lignin degradation by white-rot fungi. Understanding the molecular determinants for the interaction of this enzyme with its substrates is essential in the rational design of engineered peroxidases for biotechnological application. Here, we combine computational and experimental approaches to analyze the interaction of Phanerochaete chrysosporium LiP (isoenzyme H8) with VA and its radical cation (VA•+, resulting from substrate oxidation by the enzyme). Interaction energy calculations at semiempirical quantum mechanical level (SQM) between LiP and VA/VA•+ enabled to identify those residues at the acidic environment of catalytic Trp171 involved in the main interactions. Then, a battery of variants, with single and multiple mutations at these residues (Glu168, Asp165, Glu250, Asp264, and Phe267), was generated by directed mutagenesis, and their kinetics parameters were estimated on VA and two additional substrates. The experimental results show that Glu168 and Glu250 are crucial for the binding of VA, with Glu250 also contributing to the turnover of the enzyme. The experimental results were further rationalized through new calculations of interaction energies between VA/VA•+ and LiP with each of the single mutations. Finally, the delocalization of spin density was determined with quantum mechanics/molecular mechanics calculations (QM/MM), further supporting the contribution of Glu250 to VA oxidation at Trp171.
Keywords: Interaction energy; Lignin peroxidase; Phanerochaete chrysosporium; QM/MM; SQM; Sited-directed mutagenesis; Veratryl alcohol.
Figures



References
-
- Martinez A.T., Camarero S., Ruiz-Dueñas F.J., Martínez M.J. The royal society of chemistry. 2018. Biological lignin degradation; pp. 199–225. - DOI
