Risk of bronchospasm and coronary arteriospasm with sugammadex use: a post marketing analysis
- PMID: 31452867
- PMCID: PMC6700844
- DOI: 10.1177/2042098619869077
Risk of bronchospasm and coronary arteriospasm with sugammadex use: a post marketing analysis
Abstract
Introduction: Sugammadex is used for the reversal of neuromuscular blockade caused by rocuronium bromide and vecuronium bromide. As part of the post licensing phase of drug development, adverse events related to the use of sugammadex are still being uncovered and being reported. The potential association between sugammadex and adverse events bronchospasm and coronary arteriospasm using a retrospective pharmacovigilance signal analysis was carried out.
Methods: Food and Drug Administration's Adverse Event Reporting System database was used to run disproportionality analyses to investigate the potential association of sugammadex with bronchospasm or coronary arteriospasm. In this analysis we report the adverse event signal using frequentist methods of Relative reporting ratio (RRR), proportional reporting ratio (PRR), reporting odds ratio (ROR) and the Bayesian based Information Component metric.
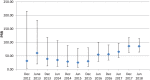
Results: A statistically significant disproportionality signal is found between sugammadex and bronchospasm (n = 44; chi-squared = 2993.87; PRR = 71.95 [95% CI: 54.00-95.85]) and sugammadex and coronary arteriospasm (n = 6; chi-squared = 209.39; PRR = 43.82 [95% CI: 19.73-97.33]) as per Evans criteria. Both statistically significant disproportionality signals persisted when stratified by gender. Based upon dynamic cumulative PRR graph, the PRR value has steadily increased and the 95% CI narrowed since December 2012.
Conclusion: The results of the pharmacovigilance analysis highlight a statistically significant disproportionality signal between sugammadex usage and bronchospasm and coronary arteriospasm adverse events. Physicians need to be aware of these adverse events when using sugammadex. The results of the pharmacovigilance signal analysis highlight a statistically significant disproportionality signal between sugammadex usage and bronchospasm and coronary arteriospasm adverse events. Physicians need to be aware of these adverse events when using sugammadex.
Keywords: Bronchospasm; Coronary Arteriospasm; Sugammadex.
Conflict of interest statement
Conflict of interest statement: The author declares that there is no conflict of interest.
Figures
Similar articles
-
A pharmacovigilance study of FDA adverse events for sugammadex.J Clin Anesth. 2024 Oct;97:111509. doi: 10.1016/j.jclinane.2024.111509. Epub 2024 Jun 15. J Clin Anesth. 2024. PMID: 38880003
-
Pyoderma gangrenosum adverse event with Rituximab use: A postmarketing pharmacovigilance analysis.Dermatol Ther. 2020 Mar;33(2):e13221. doi: 10.1111/dth.13221. Epub 2020 Jan 17. Dermatol Ther. 2020. PMID: 31925868
-
Adverse Events Associated With Radium-223 in Metastatic Prostate Cancer: Disproportionality Analysis of FDA Data Reflecting Worldwide Utilization.Clin Genitourin Cancer. 2020 Jun;18(3):192-200.e2. doi: 10.1016/j.clgc.2019.11.017. Epub 2019 Dec 5. Clin Genitourin Cancer. 2020. PMID: 31902714 Free PMC article.
-
Priapism associated with anti-seizure medications: a pharmacovigilance study and a review of published cases.Expert Opin Drug Saf. 2024 Jan;23(1):67-78. doi: 10.1080/14740338.2023.2293208. Epub 2023 Dec 11. Expert Opin Drug Saf. 2024. PMID: 38062555 Review.
-
Efficacy and safety of sugammadex compared to neostigmine for reversal of neuromuscular blockade: a meta-analysis of randomized controlled trials.J Clin Anesth. 2016 Dec;35:1-12. doi: 10.1016/j.jclinane.2016.06.018. Epub 2016 Aug 4. J Clin Anesth. 2016. PMID: 27871504 Review.
Cited by
-
Sugammadex-induced bronchospasm: a case report.J Dent Anesth Pain Med. 2023 Oct;23(5):287-291. doi: 10.17245/jdapm.2023.23.5.287. Epub 2023 Sep 27. J Dent Anesth Pain Med. 2023. PMID: 37841521 Free PMC article.
-
Photosensitivity From Avapritinib: Pharamacovigilance Analysis.JMIR Dermatol. 2022 Aug 10;5(3):e39229. doi: 10.2196/39229. JMIR Dermatol. 2022. PMID: 39475689 Free PMC article.
References
-
- Renew J, Naguib M, Brull S. Clinical use of neuromuscular blocking agents in anesthesia. UpToDate, https://www.uptodate.com/contents/clinical-use-of-neuromuscular-blocking... (2017, accessed 5 August 2018).
-
- Bevan DR. Neuromuscular blocking drugs: onset and intubation. J ClinAnesth 1997; 9(Suppl. 6): 36S–39S. - PubMed
-
- World Health Organization. WHO model list of essential medicines, http://www.who.int/medicines/publications/essentialmedicines/18th_EML.pdf (accessed 5 August 2018).
-
- Food and Drug Administration. Bridion application summary review, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/022225Orig1s000S... (accessed 5 August 2018).
LinkOut - more resources
Full Text Sources
Research Materials