Buffering of cytosolic calcium plays a neuroprotective role by preserving the autophagy-lysosome pathway during MPP+-induced neuronal death
- PMID: 31452956
- PMCID: PMC6700189
- DOI: 10.1038/s41420-019-0210-6
Buffering of cytosolic calcium plays a neuroprotective role by preserving the autophagy-lysosome pathway during MPP+-induced neuronal death
Abstract
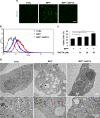
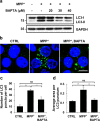
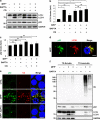
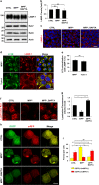
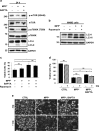
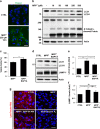
Parkinson's disease (PD) is a chronic neurodegenerative disease with no cure. Calbindin, a Ca2+-buffering protein, has been suggested to have a neuroprotective effect in the brain tissues of PD patients and in experimental models of PD. However, the underlying mechanisms remain elusive. Here, we report that in 1-methyl-4-phenylpyridinium (MPP+)-induced culture models of PD, the buffering of cytosolic Ca2+ by calbindin-D28 overexpression or treatment with a chemical Ca2+ chelator reversed impaired autophagic flux, protecting cells against MPP+-mediated neurotoxicity. When cytosolic Ca2+ overload caused by MPP+ was ameliorated, the MPP+-induced accumulation of autophagosomes decreased and the autophagic flux significantly increased. In addition, the accumulation of damaged mitochondria and p62-positive ubiquitinated protein aggregates, following MPP+ intoxication, was alleviated by cytosolic Ca2+ buffering. We showed that MPP+ treatment suppressed autophagic degradation via raising the lysosomal pH and therefore reducing cytosolic Ca2+ elevation restored the lysosomal pH acidity and normal autophagic flux. These results support the notion that functional lysosomes are required for Ca2+-mediated cell protection against MPP+-mediated neurotoxicity. Thus, our data suggest a novel process in which the modulation of Ca2+ confers neuroprotection via the autophagy-lysosome pathway. This may have implications for the pathogenesis and future therapeutic targets of PD.
Keywords: Cell death in the nervous system; Neurological disorders.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

