Analysis of low-scattering regions in optical coherence tomography: applications to neurography and lymphangiography
- PMID: 31453005
- PMCID: PMC6701530
- DOI: 10.1364/BOE.10.004207
Analysis of low-scattering regions in optical coherence tomography: applications to neurography and lymphangiography
Abstract
Analysis of semi-transparent low scattering biological structures in optical coherence tomography (OCT) has been actively pursued in the context of lymphatic imaging, with most approaches relying on the relative absence of signal as a means of detection. Here we present an alternate methodology based on spatial speckle statistics, utilizing the similarity of a distribution of given voxel intensities to the power distribution function of pure noise, to visualize the low-scattering biological structures of interest. In a human tumor xenograft murine model, we show that these correspond to lymphatic vessels and nerves; extensive histopathologic validation studies are reported to unequivocally establish this correspondence. The emerging possibility of OCT lymphangiography and neurography is novel and potentially impactful (especially the latter), although further methodology refinement is needed to distinguish between the visualized lymphatics and nerves.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures
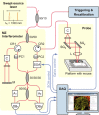



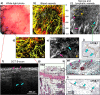
References
-
- Vakoc B. J., Lanning R. M., Tyrrell J. A., Padera T. P., Bartlett L. A., Stylianopoulos T., Munn L. L., Tearney G. J., Fukumura D., Jain R. K., Bouma B. E., “Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging,” Nat. Med. 15(10), 1219–1223 (2009). 10.1038/nm.1971 - DOI - PMC - PubMed
-
- Birim O., Kappetein A. P., Stijnen T., Bogers A. J., “Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer,” Ann. Thorac. Surg. 79(1), 375–382 (2005). 10.1016/j.athoracsur.2004.06.041 - DOI - PubMed
LinkOut - more resources
Full Text Sources
