Neuron-generated thrombin induces a protective astrocyte response via protease activated receptors
- PMID: 31453648
- PMCID: PMC7391964
- DOI: 10.1002/glia.23714
Neuron-generated thrombin induces a protective astrocyte response via protease activated receptors
Abstract
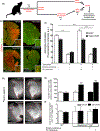
Astrocytes protect neurons during cerebral injury through several postulated mechanisms. Recent therapeutic attention has focused on enhancing or augmenting the neuroprotective actions of astrocytes but in some instances astrocytes can assume a neurotoxic phenotype. The signaling mechanisms that drive astrocytes toward a protective versus toxic phenotype are not fully known but cell-cell signaling via proteases acting on cell-specific receptors underlies critical mechanistic steps in neurodevelopment and disease. The protease activated receptor (PAR), resides in multiple brain cell types, and most PARs are found on astrocytes. We asked whether neuron-generated thrombin constituted an important astrocyte activation signal because our previous studies have shown that neurons contain prothrombin gene and transcribed protein. We used neuron and astrocyte mono-cell cultures exposed to oxygen-glucose deprivation and a model of middle cerebral artery occlusion. We found that ischemic neurons secrete thrombin into culture media, which leads to astrocyte activation; such astrocyte activation can be reproduced with low doses of thrombin. Media from prothrombin-deficient neurons failed to activate astrocytes and adding thrombin to such media restored activation. Astrocytes lacking PAR1 did not respond to neuron-generated thrombin. Induced astrocyte activation was antagonized dose-dependently with thrombin inhibitors or PAR1 antagonists. Ischemia-induced astrocyte activation in vivo was inhibited after neuronal prothrombin knockout, resulting in larger strokes. Restoring prothrombin to neurons with a lentiviral gene vector restored astrocyte activation and reduced stroke damage. We conclude that neuron-generated thrombin, released during ischemia, acts via PAR1 and may cause astrocyte activation and paracrine neuroprotection.
Keywords: astrocyte activation; ischemia; neuroprotection; thrombin.
© 2019 Wiley Periodicals, Inc.
Figures





References
-
- Bushell TJ, Cunningham MR, McIntosh KA, Moudio S, & Plevin R (2016). Protease-activated receptor 2: Are common functions in glial and immune cells linked to inflammation-related CNS disorders? Current Drug Targets, 17(16), 1861–1870. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

